ROBERT ENICK, University of Pittsburgh; JAMES AMMER and ALBERT YOST, National Energy Technology Laboratory; WILLIAM SCHULLER, URS Corporation
Carbon dioxide foam floods are typically conducted via the alternate injection of aqueous surfactant solution slugs and pure CO2 (SAG). The notion of adding surfactant to CO2 for conformance and/or mobility control was suggested decades ago. Recent developments in the identification and design of CO2-soluble surfactants that generate foams via core studies have re-kindled interest in this idea.1,2 Because oil-bearing formations initially contained brine, and decades of waterflooding have added additional fluids, the need for alternating slugs of an aqueous solution can be reduced or possibly eliminated. Further, the dissolution of surfactant in CO2 helps to ensure that surfactant will be available for in-situ foam generation in areas where the CO2 is flowing.
An operator may choose to dissolve the CO2-soluble surfactant in both the CO2 and the brine because the non-ionic surfactants that are capable of dissolving in CO2 and stabilizing CO2-in-brine foam are also water-soluble. Proprietary CO2-soluble surfactants for EOR applications are available from Dow Oil & Gas (see sidebar). An ongoing NETL-RUA (Regional University Alliance) project with the University of Pittsburgh has shown that commercially available surfactants from manufacturers such as Huntsman have also yielded promising lab-scale results in Berea, Bentheimer and SACROC cores.2,3
The Department of Energy (DOE) is currently supporting a CO2-soluble surfactant research project at the University of Texas at Austin (UTA) for application in heterogeneous carbonate and sandstone reservoirs.4 High-quality foams with very low surfactant concentrations are being designed with the goal of generating stable foams that break in the presence of oil. Nonionic alkyl ethoxylate and alkylphenol ethoxylate surfactants formed highly viscous CO2/water (C/W) foams with viscosities that were orders of magnitude higher than pure CO2 at 24°C and 70°C and unstable oil/water (O/W) emulsions, which indicate that these surfactants are selective for stable C/W foams but produce unstable O/W emulsions. The effect of shear rate and foam quality on foam viscosity was also quantified. On the basis of this research as well as field-scale simulations, Marathon is considering a CO2 foam EOR pilot in the Steamboat Butte reservoir. The UTA team is also working with Occidental Petroleum to characterize the Hobbs reservoir for a future foam process.
Water-dispersible nanoparticles for foam stabilization. Johnston and co-workers at UTA pioneered the use of water-dispersible nanoparticules (rather than water-soluble surfactants) for generating Pickering emulsions (particle-stabilized emulsions) of CO2-in-water for use as CO2 mobility control foams.5 These particles must be much smaller than the pore throats of the porous medium. Figure 1 shows that a contact angle θ of 0–90° (for a “water-wet particle”) would be expected for oil-in-water emulsions, which are analogous to CO2-in-water emulsions, to be stabilized. Foams would be generated in situ via alternating injections of aqueous nanoparticle dispersions and CO2. Fumed silica is a promising candidate due to its long-term stability, absence of adsorption losses, availability in large quantities, and ability to be coated with compounds that can alter its surface properties.6
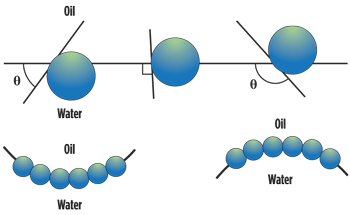 |
| Fig. 1. Schematic demonstrating the relationship between contact angle (measured through the aqueous phase) and the position of a spherical particle at the planar oil-water interface (analogous to the CO2-water interface); < 90° (left), ~ 90° (center), and > 90° (right). Also shown is the emulsion morphology based on contact angle.5 |
|
Two ongoing nanoparticle-related research projects are also being supported by DOE. A UTA team demonstrated that low-cost nanoparticles (e.g., nanoclays, fumed silica, iron oxide) can be coated to provide foam stabilizing performance similar to, and process economics comparable to or better than, that of surfactants.7 Foam generated by co‐injecting liquid CO2 (ambient temperature, 1,800 psia) and an aqueous dispersion of polyethylene glycol‐coated silica nanoparticles through a fractured sandstone core (effective fracture aperture of 40 microns) showed that the CO2 and brine foam viscosity ranged from two to five times greater than that of fluids without nanoparticles, depending on the proportions of CO2 and brine. Foams formed in fractures require a threshold shear rate, which raises the possibility of creating “self-guiding” fluids that selectively reduce CO2 mobility by generating foam only in regions, such as fractures, where CO2 is flowing rapidly.
A team at the Petroleum Recovery Research Center at the New Mexico Institute of Mining and Technology is investigating foam generation at dynamic conditions and nanosilica particle-stabilized CO2 foam transport in Berea sandstone and Indiana limestone cores.8 Initial coreflooding tests focused on nanosilica dispersions with concentrations between 4,000 and 6,000 ppm, which were injected into the cores after water flooding. Silica nanoparticles easily passed through the sandstone core without changing the core permeability, whereas little adsorption occurred in the limestone core with no permeability change.9 Current research is focused on the recovery of residual oil remaining after waterflooding.
CO2-soluble thickeners. Increasing CO2 viscosity to a value comparable to oil via the dissolution of a dilute concentration of a “CO2 thickener” has been a longstanding goal for CO2-EOR, which would allow operators to achieve a favorable mobility ratio without WAG or foams. A flurry of CO2-thickening research examining dozens of candidates occurred during the 1980s and 1990s, with investigators considering both high-molecular-weight polymers and smaller associating molecules. Most candidates were known oil-thickening compounds. Mostly, results merely documented how difficult it was to dissolve a thickener in CO2 unless very large amounts of an organic liquid co-solvent were added. Some considered compounds that readily dissolved in CO2, but the thickener had such a limited capacity to increase the viscosity that very high concentrations were required. Notable verifiable advances included the dissolution of a several percent by weight of high-molecular-weight silicone oil in CO2 with toluene as a co-solvent;10 the synthesis of high-molecular-weight polyfluoroacylates that were very CO2-soluble at EOR conditions and could thicken CO2 at several percent by weight;11 and the NETL-sponsored design and synthesis of a fluoroacylate-styrene random copolymer (polyFAST) that dissolved in CO2 at EOR conditions without the need for a co-solvent and thickened CO2 flowing through sandstone at concentrations as low as 0.2 wt%. PolyFAST was too expensive for field testing, however.12 An economic, environmentally benign CO2 thickener, which can dissolve in CO2 without the need for a co-solvent, and can reduce the mobility ratio to ~1, remains an objective with high-risk, but with high-reward.
Going forward. DOE’s Office of Fossil Energy through the National Energy Technology Laboratory (NETL) awarded a number of new research projects in 2010 seeking to advance next generation CO2-EOR to the point of pilot (small) scale testing. Three of these activities summarized above, as well as the ongoing field trial by Dow Oil & Gas and Kinder Morgan and the ongoing NETL-RUA project with the University of Pittsburgh, will hopefully bring new life to CO2 mobility control research and field applications. Based on a national resource assessment for CO2-EOR by NETL, the prize is nearly 70 billion bbl of oil.
In April, the Petroleum Services Association of Canada also revised its forecast downward by 200 wells from January, predicting there will be 13,150 wells drilled this year, up slightly from 12,850 drilled in 2011. Last, but not least, the Canadian Association of Petroleum Producers (CAPP) is now calling for a 5.8% decline in drilling, from 11,368 wells in 2011 to 10,710 wells in 2012. CAPP said that 65% of all wells drilled in Canada during 2011 were oil completions, and that percentage may finish higher this year. 
REFERENCES
1. Le, V. Q., Q. P. Nguyen and A. W. Sanders, “A novel foam concept with CO2 dissolved surfactants,” SPE paper 113370, presented at the 2008 SPE Improved Oil Recovery Symposium, Tulsa, Okla., April 19–23, 2008.
2. McLendon, W., P. Koronaios, S. McNulty, R. Enick, V. Romanov and D. Crandall, “Assessment of CO2-soluble surfactants for mobility reduction using mobility measurements and CT iImaging,” SPE paper 154205, presented at the 18th SPE Improved Oil Recovery Symposium, Tulsa, Okla., April 14–18, 2012.
3. Xing, D., B. Wei, K. Trickett, A. Mohamed, J. Eastoe, Y. Soong and R. Enick, “CO2-soluble surfactants for improved mobility control,” SPE paper 129907, presented at the 2010 SPE Improved Oil Recovery Symposium, Tulsa, Okla., April 24–28, 2010.
4. http://www.netl.doe.gov/technologies/oil-gas/Petroleum/projects/EP/ImprovedRec/FE0005902-UTA.html.
5. Dickson, J. L., B. P. Binks, and K. P. Johnston, “Stabilization of carbon dioxide-in-water emulsions with silica nanoparticles,” Langmuir Vol. 20, No. 19, 2004, pp. 7976–7983.
6. Espinosa, D., F. Caldesas, K. Johnston, S. L. Bryant, and C. Huh, “Nanoparticle-stabilized supercritical CO2, foams for potential mobility control applications,” SPE paper 129925, presented at the 2010 SPE Improved Oil Recovery Symposium, Tulsa, Okla., April 24–28, 2010.
7. http://www.netl.doe.gov/technologies/oil-gas/Petroleum/projects/EP/ImprovedRec/FE0005917-UTA.html.
8. http://www.netl.doe.gov/technologies/oil-gas/Petroleum/projects/EP/ImprovedRec/FE0005979-NMIMT.html.
9. Yu, J., C. An, D. Mo and N. Liu, “Study of adsorption and transportation behavior of nanoparticles in three different porous media,” SPE paper 153337, presented at the 2012 SPE Improved Oil Recovery Symposium, Tulsa, Okla., April 16–18, 2012.
10. Bae, J. H., and C. A. Irani, “A laboratory investigation of viscosified CO2 process,” SPE paper 20467, September 1990, published as SPE Advanced Technology Series, April 1993, Vol. 1, No. 1, pp. 166–171.
11. McClain, J. B., D. E. Betts, D. A. Canelas, E. T. Samulski, J. M. DeSimone, J. D. Landona and G. D. Wignall, “Characterization of polymers and amphiphiles in supercritical CO2 using small angle neutron scattering and viscometry,” proceedings of the 1996 Spring meeting of the ACS, Division of Polymeric Materials, Science and Engineering, Vol. 74, pp. 234–235, New Orleans, La.
12. Xu, J., A. Wlaschin and R.Enick, “Thickening carbon dioxide with the fluoroacrylate-styrene copolymer,” SPEJ, June 2003, pp. 85–91.
13. Sanders, A., R. Jones, T. Mann, L. Patton, M. Linroth, and Q. Nguyen, “Successful implementation of CO2-foam for conformance control,” slide presentation at the 2010 CO2 Conference, Midland, Texas, Dec. 9–10, 2010.
|
The author
|
 |
ROBERT ENICK is the Bayer Research Professor of Chemical and Petroleum Engineering at the University of Pittsburgh, and has worked in various capacities with NETL scientists since 1987. He has developed numerous compounds designed to dissolve in CO2, including direct CO2 thickeners, for over 20 years. Dr. Enick led the team that designed the fluoroacrylate-styrene copolymer (polyFAST) direct thickener—the only compound identified to date capable of increasing CO2 viscosity by a factor of ~10 at a concentration of ~1wt% at minimum miscible pressure (MMP) conditions without the need for a co-solvent. In recent years he has identified numerous commercially available, non-ionic, CO2-soluble surfactants with the potential to form CO2-in-brine mobility control foams in-situ as CO2-surfactant solution is injected. |
| |
|
 |
JAMES AMMER is the director of the Natural Gas & Oil Project Management Division at the National Energy Technology Laboratory (NETL), which manages external R&D projects funded through the Department of Energy’s Office of Fossil Energy Natural Gas and Oil Program. Previously he served as a project manager for 10 years, managing projects in drilling, stimulation, production optimization, natural fracture detection and prediction, and gas storage. Ammer also conducted reservoir engineering and simulation studies for over 10 years, including studies on CO2 flooding, gas migration analysis, horizontal drilling evaluation and gas storage efficiency. He received his BSc degree in Petroleum and Natural Gas Engineering from Pennsylvania State University in 1983. Ammer has been employed at NETL for over 27 years. |
| |
|
 |
ALBERT YOST is technology manager for the Traditional Oil and Gas Program at the Department of Energy’s National Energy Technology Laboratory (NETL) in Morgantown, W.Va. He serves as oil and gas technical advisor to the director, Strategic Center for Oil and Gas at NETL, and also manages a multi-million-dollar portfolio of research projects focused on accelerating the recovery of domestic unconventional oil and natural gas resources, EOR, and mitigating environmental impact. Mr. Yost has more than 35 years of experience in management of federal research related to horizontal drilling and stimulation; EOR using CO2; natural gas storage, transmission, and distribution; and water-related environmental research. He has published more than 30 papers related to unconventional gas and EOR. He received a BS in petroleum engineering in 1975 and an MS in petroleum engineering in 1982 from West Virginia University. |
| |
|
 |
WILLIAM SCHULLER is a senior scientist with URS Corporation (Energy and Construction Services Division, Global Management and Operations Services Business Unit) providing technical support to DOE’s National Energy Technology Laboratory’s (NETL) Office of Research and Development. Additionally, he is the Oil and Gas Program Lead for Team KeyLogic (KeyLogic, Inc. and URS joint venture) providing project execution and integration services support to the Natural Gas and Oil Project Management Division at NETL. Schuller has a BS in Geology from West Virginia University and has over 35 years of oil and gas experience in reservoir characterization and production enhancement. |
|