
DR. ROBERT ENICK, University of Pittsburgh; JAMES AMMER and ALBERT YOST, National Energy Technology Laboratory; WILLIAM SCHULLER, URS Corporation
The density of carbon dioxide at CO2-EOR reservoir conditions is 0.4–0.8 g/cm3, which is typically less than oil and brine, resulting in CO2 gravity override. The viscosity of CO2 at typical EOR conditions is 0.04–0.08 cP, which is very low relative to brine and oil, and promotes viscous fingering, exacerbates gravity override, and leads to early breakthrough and high ratios of CO2 to produced oil. The CO2 density cannot be significantly increased at CO2-EOR operating conditions. However, several strategies greatly reduce CO2 mobility—most commonly, the water-alternating-gas (WAG) process. The WAG process increases water saturation and decreases CO2 saturation, thereby reducing the relative CO2 permeability. Field studies where 80% hydrocarbon pore volume CO2 had been injected indicated that WAG floods leave behind about one-third to two-thirds of the oil remaining after waterflooding.1
As a more effective means of reducing CO2 mobility, many researchers promoted using CO2 foams by simultaneously injecting CO2 and surfactant solution or by injecting alternating slugs of aqueous surfactant solutions and CO2 (SAG). The mobility of CO2 is reduced by the in situ generation of surfactant-stabilized, mobile lamellae formed by the leave-behind, lamella division and snap-off mechanisms.2-4 Mobile lamellae bridge pore throats and encapsulate pockets of dense CO2, thereby reducing the CO2 relative permeability, while immobile lamellae formed during this process can trap very small bubbles of CO2 within some portion of the porous media, Fig. 1.5-7
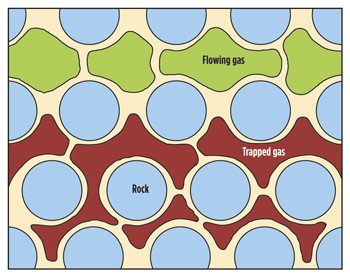 |
| Fig. 1. Foam flow in 2-D porous media. Light blue = water-wet sand grains (shown un-cemented for clarity); white = surfactant solution; dark red = trapped CO2 with small pore constrictions and channels; light green = flowing CO2 cells in larger pore channels separated by surfactant-stabilized lamellae.9 |
|
Laboratory-based foams were extensively studied due to the relative ease of achieving substantial reductions in mobility using dilute concentrations (~0.01–1.0wt%) of readily available detergents. Most ionic surfactants were anionic because sandstone surfaces and most carbonates are negatively charged and surfactant loss to adsorption was undesirable. Cationic surfactants were used for relatively uncommon positively charged carbonates. Numerous surfactants stabilized foams in cores including anionic, nonionic, zwitterionic, cationic and some proprietary mixture surfactants.
Relatively simple, low-pressure screening tests provided initial CO2 foam stability assessments, including the determination of surfactant solubility in brine at reservoir conditions, the decrease in the water surface tension caused by a surfactant, and the stability of a foam formed by mixing a low-pressure gas and a soap solution. The chemical stability of the surfactant itself was assessed in long-term tests to determine chemical composition changes after prolonged periods at reservoir temperature. Surfactant adsorption losses on the rock surfaces were estimated, particularly ionic surfactants at high concentrations. Adsorption losses were conducted over a very wide range of surfactant concentrations because CO2 foams can be generated at surfactant concentrations below and above the critical micelle concentration (cmc).
To understand foam formation mechanisms in porous media, researchers observed the invasion of a gas into a two-dimensional, low-pressure glass micro-model with the pore space initially filled with surfactant solution.7 The relative permeability of gas and water was measured in unconsolidated and consolidated porous media. Tracer gas analysis and computed tomography (CT) imaging measured mobile gas and trapped gas saturations of foam within a core. Scanning electron microscopy (SEM) images showed surfactant-stabilized lamellae bridging pore throats, Fig. 2.
 |
| Fig. 2. A low-energy SEM image of a broad, undulating sheet-like lamella covering a number of pore entrances.10 |
|
Promising low-pressure surfactant screening tests were assessed in high-pressure experiments for a more realistic picture of the CO2-EOR process at reservoir conditions. High-pressure interfacial tension (IFT) measurements were made on an aqueous/CO2 phase interface to determine the cmc of the surfactant and the extent of IFT reduction over a wide range of surfactant concentrations.8 Numerous unconsolidated and consolidated porous media studies were conducted. Viscous fingering suppression and the plug-like displacement of brine by CO2 that occurs when foam forms were observed in CT images. Constant quality foam properties were established using steady-state mobility measurements involving simultaneous injections of CO2 and surfactant solution into cores. Numerous foam mobility studies determined the influence of surfactant type, surfactant concentration, brine composition, foam quality, rock wettability, permeability, oil saturation, superficial velocity, temperature and pressure. Other studies showed that foams did not damage formations, could be readily dissipated if necessary by injection of large amounts of brine, and could reduce CO2 mobility by roughly one to three orders of magnitude at surfactant concentrations of ~0.1–1.0wt%. The most significant mobility decreases were associated with high surfactant concentration, high permeability media, low oil saturation, foam quality in the ~60–80% CO2 range, and superficial velocities of more than ~1 ft/day.
Lab-scale CO2 foams showed the potential for conformance control. High-permeability thief zones were blocked with very strong CO2 foam diverting subsequently injected CO2 into un-swept, lower perm, oil-bearing layers. Most dual, parallel core systems involved the development of CO2 injection strategies in layered formations. Foams could also be designed for mobility control by tailoring their apparent viscosity to be comparable to that of oil and brine, thus improving volumetric sweep in targeted oil recovery areas. To substantiate mobility control, displacements simulating tertiary CO2 floods (with and without surfactant) were conducted. Oil displacement by CO2 foam across single cores studied pressure drops across the core at a given flowrate, monitored oil recovery, and compiled displacement process CT images.
Examples cited represent a much larger body of evidence pointing to CO2 foams as a viable means of conformance and/or mobility control. To summarize:
-
Many commercially available water-soluble surfactants are capable of generating CO2-in-brine foams.
-
Porous media are not damaged by CO2 foams, and brine injections readily dissipate the foams.
-
Carbon dioxide mobility can be reduced with CO2 foams by as much as three orders of magnitude.
-
Viscous fingering can be suppressed with CO2 foams designed for improved mobility control.
-
Carbon dioxide conformance control foams can effectively block a watered-out high-perm core and divert subsequently injected CO2 to a low-perm, oil-bearing core.
-
Surfactant adsorption will always occur, but adsorptive losses can be mitigated via the selection of an appropriate type and concentration of surfactant.
-
High-pressure CO2 foams can be generated in the lab via the simultaneous injection of CO2 and an aqueous surfactant solution, or via a surfactant solution-alternating-CO2 gas (SAG) injection sequence.
-
Low mobility CO2 foams are usually more readily formed at high surfactant concentration, high fluid superficial velocity, foam quality of ~60–80%, and low oil saturation conditions.
Part 3 of this series will summarize more than a dozen CO2 foam pilot test results, and discuss the simultaneous emergence of gels as a competing conformance control technology. 
LITERATURE CITED
1. Merchant, D. H., “Life Beyond 80: A Look at Conventional WAG Recovery Beyond 80% HCPV Injection in CO2 Tertiary Floods,” SPE 139516, presented at the SPE International Conference on CO2 Capture, Storage and Utilization, New Orleans, Louisiana, Nov. 10–12, 2010. Also presented at the 2010 CO2 Conference, Midland, Texas, Dec. 9–10, 2010.
2. Nguyen, Q. P., A. V. Alexandrov, P. L. Zitha, and P.K. Currie, “Experimental and Modeling Studies on Foam in Porous Media: A Review,” SPE 58799, prepared for presentation at the 2000 SPE International Symposium on Formation Damage Control, held in Lafayette,
Louisiana, February 23–24, 2000.
3. Chen, M., Y. C. Yortsos, and W. R. Rossen, “A Pore-Network Study of the Mechanisms of Foam Generation,” SPE 90939, presented at the SPE Annual Technical Conference and Exhibition, Houston, Texas, Sep. 26–29, 2004.
4. Chambers, K. T., and C. J. Radke, “Micromodel Foam Flow Study,” unnumbered report, prepared for U.S. Department of Energy, University of California, Chemical Engineering Department, October 1990.
5. Chen, Q., M. G. Gerritsen, and A. R. Kovscek, “Modeling Foam Displacement with the Local Equilibrium Approximation: Theory and Experiment Verification,” SPE 116735, presented at the 2008 SPE Annual Technical Conference and Exhibition, Denver, Colorado, Sep. 21–24, 2008.
6. Kovscek, A. R., and C. J. Radke, “Fundamentals of Foam Transport in Porous Media,” Foams: Fundamentals and Applications in the Petroleum Industry, L.L. Schramm (ed.) ACS Adv. Chem. Ser., ACS, Washington, DC, Vol. 3, No. 242, 1994.
7. Kuhlman, M. I., “Visualizing the Effect of Light Oil on CO2 Foams,” JPT, July 1990, pp. 902–908.
8. Adkins, S.S., X. Chen, Q. P. Nguyen, A. W. Sanders, and K. P. Johnston, “Effect of Branching on the Interfacial Properties of Nonionic Hydrocarbon Surfactants at the Air-Water and Carbon Dioxide-Water Interfaces,” Journal of Colloid and Interface Science, Vol. 346, 2010, pp. 455–463.
9. Radke, C., and J. Gillis, “A Dual Gas Tracer Technique for Determining Trapped Gas Saturation during Steady Foam Flow in Porous Media,” SPE 20519, presented at the 65th Annual Technical Conference and Exhibition of the SPE, New Orleans, Louisiana, Sep. 23–26, 1990.
10. Kutay, S. M., and L. L. Schramm, “Structure/Performance Relationships for Surfactant and Polymer Stabilized Foams in Porous Media,” JPCT, February 2004, Vol. 43, No. 2, pp.19–28.
|
AUTHORS
|
 |
DR. ROBERT ENICK is the Bayer Research Professor of Chemical and Petroleum Engineering at the University of Pittsburgh, and has worked in various capacities with NETL scientists since 1987. He has developed numerous compounds designed to dissolve in CO2, including direct CO2 thickeners, for over 20 years. Enick led the team that designed the fluoroacrylate-styrene copolymer (polyFAST) direct thickener—the only compound identified to date capable of increasing CO2 viscosity by a factor of ~10 at a concentration of ~1wt% at minimum miscible pressure (MMP) conditions without the need for a co-solvent. In recent years he has identified numerous commercially available, non-ionic, CO2-soluble surfactants with the potential to form CO2-in-brine mobility control foams in-situ as CO2-surfactant solution is injected. |
|
 |
JAMES AMMER is the Director of the Natural Gas & Oil Project Management Division at the National Energy Technology Laboratory (NETL), which manages external R&D projects funded through the Department of Energy’s Office of Fossil Energy Natural Gas and Oil Program. Previously he served as a project manager for 10 years, managing projects in drilling, stimulation, production optimization, natural fracture detection and prediction, and gas storage. Ammer also conducted reservoir engineering and simulation studies for over 10 years, including studies on CO2 flooding, gas migration analysis, horizontal drilling evaluation and gas storage efficiency. He received his BSc degree in Petroleum and Natural Gas Engineering from Pennsylvania State University in 1983. Ammer has been employed at NETL for over 27 years. |
|
 |
ALBERT YOST is Technology Manager for the Traditional Oil and Gas Programa at the Department of Energy’s National Energy Technology Laboratory (NETL) in Morgantown, W.Va. He serves as oil and gas technical advisor to the director, Strategic Center for Oil and Gas at NETL, and also manages a multi-million-dollar portfolio of research projects focused on accelerating the recovery of domestic unconventional oil and natural gas resources, EOR, and mitigating environmental impact. Mr. Yost has more than 35 years of experience in management of federal research related to horizontal drilling and stimulation; EOR using CO2; natural gas storage, transmission, and distribution; and water-related environmental research. He has published more than 30 papers related to unconventional gas and EOR. He received a BS in petroleum engineering in 1975 and an MS in petroleum engineering in 1982 from West Virginia University. |
|
 |
WILLIAM SCHULLER is a Senior Scientist with URS Corporation (Energy and Construction Services Division, Global Management and Operations Services Business Unit) providing technical support to DOE’s National Energy Technology Laboratory’s (NETL) Office of Research and Development. Additionally, he is the Oil and Gas Program Lead for Team KeyLogic (KeyLogic, Inc. and URS joint venture) providing project execution and integration services support to the Natural Gas and Oil Project Management Division at NETL. Schuller has a BS in Geology from West Virginia University and has over 35 years of oil and gas experience in reservoir characterization and production enhancement. |
|









