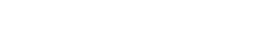|
Vol. 232 No. 6 |

|
|
HENRY TERRELL, CONTRIBUTING NEWS EDITOR
|
Learning to get along with NORM and the bone-seekers
 |
Temperature and pressure changes in formation water can lead to a buildup of radium-rich scale on the inner walls of production equipment. Image courtesy of Fugro-HGN.
|
|
There is an upside to NORM (naturally occurring radioactive material). If it wasn’t here, we might not be here. Radioactive decay keeps the Earth’s interior hot and fluid, which allows mantle convection, which creates a dynamic world and a magnetic field that protects us from deadly cosmic rays, allowing life as we know it to flourish. Now that’s an upside.
After the question of the world’s age ceased to be theological, it was generally accepted that the Earth was very old. Estimates—based on erosion, sedimentation and other observable processes—varied greatly, from tens of thousands of years to hundreds of millions. A practical upper time limit was imposed by the understanding that the interior was hot, compressed and partially melted by the force of gravity as the world formed. If the planet was more than, say, half a billion years old, it was thought, then it would have cooled down by now. Lord Kelvin estimated the Earth’s age at about 100 million years, based on how much heat remained. If it was much older than that, another heat source would be required.
Lord K knew nothing about NORM. Its discovery provided an important piece of the puzzle. Elements present in rock since the world coalesced undergo atomic decay, slowly, and emit significant heat in the process. The insulation of the Earth’s crust keeps the heat from escaping.
Radionuclide roll call. There are a lot of radioactive isotopes in the universe, but the ones that matter for us are the long-lived ones: potassium, thorium and uranium. When the Earth was molten, most of these heavy elements sank deep into the interior. They are present in the crust only in minute quantities, and human exposure is rare.
Unfortunately, atomic decay doesn’t directly lead to a stable isotope, but to radioactive “daughter” products such as radium and radon gas. These, when breathed or otherwise ingested, can be deadly. Radon gas is said to be the second leading cause of lung cancer in the US, after smoking. Worse, radium’s various forms and decay products are called “bone-seeker radionuclides” because of their tendency to adhere to the chlorides in bone tissue. Radium emits alpha particles, which can penetrate only a short distance. However, it can do considerable damage when lodged inside the body and given enough time. Radium 226 has a half-life of 1,600 years. It has time.
Radium and radon are small atoms that easily fit in the nooks and crannies of more complex compounds, particularly sulfates and salts. When these substances, with radium atoms aboard, are present in produced fluids from reservoirs, whether water, drilling mud, sludge or flowback from hydrofracing, contaminants can collect in pits and evaporation ponds. The two main forms of radium (226 and 228) can build up to form technologically enhanced NORM, or TENORM. Radium can also concentrate in scales that form in pipes and storage tanks (see photo).
Regulation. Radiation levels in equipment and produced fluids are not regulated by the US Nuclear Regulatory Commission, which sets nuclear waste disposal standards. Federal standards for exposure are established by the EPA, but disposal regulation is up to the states.
The Texas Railroad Commission has set a standard for safe individual exposure at 100 millirems/yr. (REM is an acronym for Röntgen Equivalent Man. Wilhelm Röntgen was discoverer of the X-ray. Now you know.) You get about 35 millirems/yr background radiation from space if you’re at sea level.
Mitigation. NORM waste can be disposed of legally by 1) sealing it permanently below the water table in a plugged and abandoned well, or 2) burying it or diluting it with soil until the dirt is at a safe emission level below 30 pCi/g (picocurries per gram). For comparison, 5 pCi/g is regarded as the safe limit for drinking water.
Just as dental technicians are the ones taking the risk from dental X-rays, it’s the workers that clean up radium waste and recycle contaminated equipment who risk NORM exposure. Ingesting radon gas and radioactive dust can be prevented by completely pedestrian and time-tested methods: dust masks, respirators, decontaminating clothing and tools.
Both government agencies and specialists in the radiation-protection field have been pushing for adoption of international standards for NORM in the oil field. In the past, the problem has taken a back seat to more pressing safety issues. An International Atomic Energy Agency review published a few years back stated simply: “Exposures to natural sources [of radiation] are in most cases not a matter for regulatory concern.” This view is changing recently, particularly as the industry has met pushback from citizens’ groups and environmental organizations over increased use of hydrofracing. Clearly, internationally adopted NORM standards are in everyone’s interest. It’s not enough to say it’s not a big problem. The very word “radiation” can generate a lot of heat. 
henry.terrell@gulfpub.com
|