Membrane-based desalination process treats steamflooding produced water
The treatment produces high-quality water suitable for aquifier recharge with low waste volumes.
The treatment produces high-quality water suitable for aquifier recharge with low waste volumes.Charles Webb, Chevron North America Exploration & Production; Lnsp Nagghappan, Gerald Smart and John Hoblitzell, Veolia Water; and Rich Franks, Hydranautics/Nitto Denko
San Ardo Field in California produces heavy oil via steamflooding. The process typically results in the production of 10 or more barrels of water for every barrel of oil recovered. Historically, a portion of this water has been recycled and softened to provide water for steam generation, with the remainder going to local EPA class II injection wells for disposal. However, the injection zone capacity is limited, which has constrained full field development. In October 2007, a desalination facility was commissioned to allow a portion of the produced water to be treated and discharged to the shallow freshwater aquifer, thereby allowing field development to progress. The treatment system consists of deoiling followed by a proprietary treatment train incorporating degasification, chemical and ion exchange softening, multimedia and cartridge filtration, double-pass reverse osmosis, pH neutralization and partial remineralization. This technology, portions of which were developed jointly and patented separately by Chevron USA Inc. and Veolia Water Solutions and Technologies’ N.A. Water Systems, has proven to be reliable and robust for successfully treating produced water for surface discharge. INTRODUCTION San Ardo Field comprises about 2,500 acres of land adjacent to the Salinas River in Monterey County, California, about 50 mi north of San Luis Obispo. With an estimated ultimate oil recovery of 530 million bbl, it is the 13th-largest oil field in California.1 It was discovered in 1947 and is operated in two separate units by Chevron North American Exploration and Production and Aera Energy LLC, respectively. The field is an anticlinal structure with two productive zones: the Aurignac and Lombardi sands. These zones are part of the Monterey Formation, a sedimentary rock unit that underlies much of coastal California. In places, the sands are several hundred feet thick and contain abundant quantities of 11–13°API crude. The average formation depth ranges from 1,800 to 2,200 feet below ground level. Steamflooding has been used continuously for oil recovery in the Aurignac and Lombardi sands since the 1960s; the process involves injection wells forcing steam into the formation to heat the crude and decrease its viscosity. However, oil production has been declining in recent years due to limited capacity for disposing of the produced water, which can range from 10 to 20 times the oil production rate. The challenge for producing the remaining heavy oil, particularly in the Lombardi sand, is to remove the excess water from the reservoir. Dewatering reduces the formation pressure, thereby allowing the injected steam to contact the remaining heavy oil for production. Dewatering, in essence, would allow the area of steam-enhanced production to be expanded. In the early 1990s, several alternatives for dewatering the reservoir were evaluated. The most promising alternatives were a) transporting the water to depleted oil fields in the San Joaquin Valley and b) desalination followed by surface discharge to the local groundwater aquifer. Disposal in San Joaquin Valley fields was eliminated due to a number of factors and uncertainties including economic and regulatory issues. Two desalination options were considered: mechanical vapor compression and reverse osmosis. RO was eventually selected on the basis of a successful field trial conducted at San Ardo in the early 1990s.2 Since then, additional field trials and bench scale tests have verified the efficacy of the RO technology to treat the San Ardo produced water. The desalination facility was designed to process 66,700 bpd of produced water with a recovery factor of 75%, yielding an effluent treated water rate of 50,000 bpd. This particular factor was selected based on the results of bench-scale testing and economic evaluations. The facility discharges to post-treatment constructed wetlands, which provide hydraulic equalization and quality assurance of the water prior to final disposition in groundwater recharge basins. A Waste Discharge Requirements (WDR) permit issued by the California Regional Water Quality Control Board, Central Coast Region, in 2005, covers the desalination facility, wetlands and recharge basins. Construction began in 2006 and full capacity was achieved in November 2007, Fig. 1.
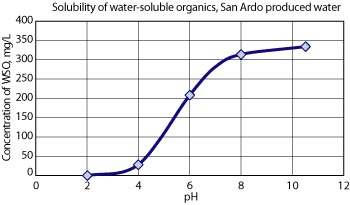
TREATMENT CHALLENGES RO membrane performance is dependent on a number of factors, including water quality, feed pressure, feed temperature, recovery, membrane construction and the presence of contaminants that can foul or scale the membranes. San Ardo produced water contains a number of these contaminants, including free oil, water-soluble organics, suspended solids, sulfides, hardness, silica and other dissolved solids, Table 1. For treatment to succeed, membrane fouling and scaling must be controlled so that recovery will be limited primarily by osmotic pressure and not by the presence of contaminants. Furthermore, the system must be sufficiently robust to handle variations in feed water quality and be capable of maximizing recovery by recycling a portion of the waste streams to the front end of the treatment process. And finally, the process must reliably treat the water to meet the stringent WDR effluent specifications, Table 2. Membrane fouling potential. The produced water exhibits high fouling potential upon concentration due to the presence of contaminants such as Water-Soluble Organics (WSOs), free oil and particulates. As a result, it is necessary to provide conditions that would remove or retain these contaminants in a soluble form to prevent fouling across the membranes. The solubility of WSOs increases with increasing pH for the San Ardo produced water, which indicates that the fouling due to organics can be controlled by operating the membrane process at an elevated pH. Additionally, the produced water contains up to 60 mg/L of free/dispersed oil. To mitigate particulate fouling of the membranes, the free oil must be reduced to less than 1 mg/L in the RO feed. Membrane scaling potential. The produced water exhibits high scaling potential upon concentration due to the presence of contaminants such as silica, calcium salts (calcium carbonate, calcium sulfate) and metal salts (iron hydroxide). Thus, it is necessary to provide conditions that would remove or retain these contaminants in a soluble form to prevent scaling across the membranes. The solubility of silica increases with increasing pH, indicating that silica scaling can be controlled by operating the membrane process at elevated pH. The technology must also control potential scaling due to calcium salts and metal precipitates by providing contaminant reduction processes such as chemical softening and ion exchange softening ahead of the RO system. Gas emission potential. The produced water contains sulfides in concentrations up to 100 mg/L, which under acidic or near-neutral pH conditions could result in the evolution of hydrogen sulfide (H2S) gas and consequently pose a hazard to personnel and the environment. Therefore, it is necessary to control potential H2S gas emissions. The concentration of free H2S gas decreases with increasing pH. This is due to the conversion of the H2S gas into bisulfide ionic form, and indicates that H2S gas emissions can be controlled by operating the treatment process at elevated pH. For unit operations that are designed to be operated at near-neutral pH, proper containment and recovery systems must be provided to prevent releases to the environment. Boron removal. The produced water contains up to 30 mg/L of boron, which must be reduced 97.5% to less than 0.75 mg/L for effluent discharge. Boron is normally present in water as boric acid, B(OH)3, which is poorly rejected by reverse osmosis membranes because of its lack of charge and small size.3 However, at elevated pH (> 10), boron exists mainly as the larger and negatively charged borate ion BOH4-, for which rejection rates for single-pass membranes can exceed 95%. Therefore, the key to boron removal is to operate the membranes at an elevated pH. This process was discovered and subsequently patented by researchers at Texaco Exploration and Production Inc. (now Chevron) in 1993.4 Feed water temperature. The produced water temperature ranges between 160 and 200°F. Typically, RO membranes are rated for 120°F or lower. Higher temperatures can result in lower salt rejection and permanently damage the membranes due to swelling. Therefore, it is necessary to cool the produced water to make it suitable for membrane-based treatment. PROCESS DESCRIPTION The field produces up to 180,000 bwpd, which is treated in an oil dehydration facility to remove the bulk of the oil for sales to pipeline. The water is then transported to the water reclamation facility (Fig. 2), where a portion is sent directly to disposal in class II injection wells. The remainder is deoiled by induced gas flotation followed by walnut shell filtration. The deoiled water is then treated by Veolia Water Solutions and Technologies’ proprietary OPUS (Optimized Pretreatment and Unique Separation) technology to make the water suitable for aquifer discharge. This technology consists of multiple treatment steps involving cooling, degasification, chemical softening, media filtration, ion exchange softening, cartridge filtration, double-pass reverse osmosis, pH neutralization and remineralization. The pretreatment processes ahead of the RO membranes are designed to reduce free oil, suspended solids, hardness and metals in the feed water. The RO process operates at elevated pH, which effectively controls biological, organic and particulate fouling; reduces silica scaling; and increases the rejection of silica and boron.
The induced gas flotation and walnut shell filtration reduce the free oil to less than 1 ppm. The deoiled water is cooled to 165°F from 200°F, and is degasified with the addition of acid to partially remove dissolved carbon dioxide and hydrogen sulfide and excess alkalinity. The degasification process helps reduce the quantity of solids generated in the downstream chemical softening process and the alkali demand associated with raising the pH. The degasified water is softened in a multistage chemical softening process for the removal of hardness and suspended solids. This softening process generates dense crystalline solids (10–15% by weight), which are settled in a clarifier and dewatered in a filter press for disposal. The water from the multistage chemical softening process is cooled to 100°F and further treated with multimedia filtration, weak acid cation ion exchange softening (in sodium form) and cartridge filtration, all at elevated pH, to further reduce hardness, metals and particulate concentrations. The pretreated water is pressurized through a double-pass RO system, operated at elevated pH, to reduce the total dissolved solids, silica, boron and organics present in the feed water. A portion of the RO permeate is used for makeup to the cooling tower to offset evaporation and blowdown. The tower circulates cooling water to heat exchangers in the desalination facility to cool the produced water. The remaining permeate is neutralized with sulfuric acid and carbon dioxide, and calcium chloride is injected to control the sodium adsorption ratio, which is a measure of the water’s suitability for agricultural use. The treated water is then discharged to the aquifer recharge basins via post-treatment constructed wetlands. The wetlands use a clay liner to prevent percolation of water into the aquifer and are planted with native flora, such as cattail. Liquid wastes consisting of softener spent regenerant, RO reject and cooling tower blowdown are disposed of in class II injection wells, and solid wastes (about 50–60% dewatered chemical sludge cake) are disposed of in a commercial landfill. Wastewater and sludge recycle/reuse are practiced throughout the process to maximize recovery and minimize waste. SYSTEM PERFORMANCE Table 2 shows typical actual water qualities for the produced water, second-pass permeate and final treated effluent as compared with the WDR permit effluent specifications. As can be seen, the effluent water quality comfortably meets the effluent specifications, particularly for TDS, sodium, chloride, nitrate and boron. The concentrations for these contaminants have remained relatively stable over time. The feed water and permeate flowrates from the RO skids have also been consistent, resulting in a constant recovery of 80.5% in the first pass and 90% in the second pass. However, feedwater conductivity (which is a proxy for TDS) into the first-pass RO membranes has risen 6–8% since startup. This increase has been mirrored by a corresponding rise in first-pass feed water pressure and permeate conductivity. Despite these changes, salt passage through the first-pass RO membranes has remained stable at about 3.5%, and the second-pass feed water pressure and permeate conductivities have not increased. As a side note, the first-pass RO membranes have rarely needed cleaning. Typical cleaning frequencies are once every 3–6 months. The second-pass RO membranes have never been cleaned. Membrane testing. After 8 months of operation, two membranes from the first-pass RO skids were removed and sent to the manufacturer for testing and autopsy. One membrane was located in the first stage of the first pass; the second was located in the second stage of the first pass. No membranes from the second-pass RO skids were tested. Flux rate and salt passage. The two membranes were tested in the laboratory at the manufacturer’s standard conditions of 225 psi and 15% recovery on a 1,500-mg/L sodium chloride feed at 25°C. The results were compared to the manufacturer’s original factory wet test, and indicated a slight flux loss in the stage-1 membrane and a larger flux loss in the stage-2 membrane. In like manner, salt passage increased slightly in the stage-1 membrane, but more in the stage-2 membrane. Differential pressures for both membranes were normal at about 4 psi. The excellent test performance of the first-stage membrane suggests that the pretreatment for the RO system is successfully removing suspended solids and colloidal mater. The test performance of the second-stage membrane suggests the presence of mineral scaling. Autopsy. After testing, the two membranes were autopsied and visually inspected. The membranes were covered with a layer of brown foulant, which was easily wiped away with a wet cloth and emitted a strong hydrocarbon odor. The foulant was scraped from the surface of each membrane and tested for Weight Loss on Ignition (WLOI) to determine organic content. The WLOI test indicated that the foulant on the stage-1 membrane was 79% organic and on the stage-2 membrane foulant was 36% organic. The membrane samples were then photographed with a scanning electron microscope, which indicated two very different foulants on the stage-1 and stage-2 membranes. An amorphous, uniform cake layer covers the membrane from stage 1. The appearance of this foulant is characteristic of colloidal or organic fouling. The stage-2 membrane was covered with a granular foulant characteristic of silica and iron scaling. The membranes were tested with Energy Dispersive X-ray (EDX) analysis to determine foulant composition. The EDX provided a quantitative confirmation of what appears in the microscope photos. The EDX for the first stage membrane revealed only trace amounts of silicon (2.4%) and iron (5.9%), but significant amounts of carbon (59%). The second-stage membrane revealed much higher quantities of silicon (28.9%) and iron (11%). Although the autopsy revealed fouling on both the first- and second-stage membranes, the flow test data showed the first-stage fouling to be minimal, suggesting that the RO pretreatment is successfully removing colloidal and particulate matter. The high-pH environment in the RO is also controlling organic fouling. The scaling on the second-stage membrane may have been caused by improper flushing of piping systems upstream of the RO membranes during commissioning; process upsets during startup and commissioning; occasional disruptions in anti-scalant dosing; or an inadvertent drop in pretreatment process pH during normal operation. Any of these events could have caused silica or other scalants to come out of solution after being concentrated in the second stage. Iron, which is a catalyst for the precipitation of silica, is also present in the water and was found on the membranes. Operational challenges. The facility faced a number of issues that affected reliability, operability and safety. The main issue has been fouling of the heat exchangers that cool water downstream of the warm lime process. These exchangers, of the plate-and-frame design, frequently foul due to calcium carbonate particles carried over in the water from the clarifier. This places the RO membranes at risk of damage due to excessively high temperature, and has resulted in frequent shutdowns and cleaning of the exchangers with acid to restore cooling capability. This problem was resolved with the installation of back-washable strainers upstream of the exchangers. Slurry handling, specifically for alkalis and clarifier sludge, has proven to be a major source of downtime and maintenance expense. The slurries damage pumps, particularly seals, and plug piping, which then requires disassembly and hydro-blasting to clear the lines and return them to normal operation. The facility is evaluating a change to more slurry-tolerant pumps and implementing controls to measure and maintain slurry concentrations below the solidification threshold. Finally, chemical handling has been a safety issue, particularly for sulfuric acid, whose corrosion characteristics change dramatically with dilution and temperature. The facility uses 93% sulfuric acid, which is relatively benign to metals from a corrosion standpoint at ambient temperatures. However, when the acid is subsequently diluted with water in the process, it can become hot and aggressive toward most metals. The problem has been resolved by lining piping and valves in sulfuric acid service with Teflon and switching to nickel-based alloys, both of which are relatively impervious to attack by dilute and concentrated sulfuric acid at temperatures up to 350°F. LITERATURE CITED 1 California Department of Conservation, “Oil and Gas Statistics,” Annual Report, Dec. 31, 2006.
|
|||||||||||||||||||||||||||||
- What's new in production (February 2024)
- Prices and governmental policies combine to stymie Canadian upstream growth (February 2024)
- U.S. operators reduce activity as crude prices plunge (February 2024)
- U.S. producing gas wells increase despite low prices (February 2024)
- U.S. drilling: More of the same expected (February 2024)
- U.S. oil and natural gas production hits record highs (February 2024)
- Applying ultra-deep LWD resistivity technology successfully in a SAGD operation (May 2019)
- Adoption of wireless intelligent completions advances (May 2019)
- Majors double down as takeaway crunch eases (April 2019)
- What’s new in well logging and formation evaluation (April 2019)
- Qualification of a 20,000-psi subsea BOP: A collaborative approach (February 2019)
- ConocoPhillips’ Greg Leveille sees rapid trajectory of technical advancement continuing (February 2019)