Ceramic membranes for produced water treatment
Already widely used in other industries, these materials are being investigated for oilfield use as a more-robust alternative to polymer membranes.Katie L. Benko, US Interior Department, Bureau of Reclamation In the Western US, development of unconventional oil and gas resources is increasing, generating large volumes of water—over 200 million gal/day.1 This water, if treated to appropriate water quality standards, could be used for beneficial purposes to augment conventional water supplies, including stream flow augmentation, irrigation, municipal uses and industrial uses. The main classifications of produced water treatment technologies are a) organic contaminant and suspended solids removal and b) desalination. Organic contaminant and suspended solids removal technologies include biological aerated filters, media filtration, settling ponds, dissolved air flotation, hydrocyclones and ultrafiltration or microfiltration. Desalination technologies include thermal technologies like distillation and vapor compression and membrane technologies like reverse osmosis and nanofiltration. In produced water applications, ultrafiltration is often used as a pretreatment for desalination. Produced water ultrafiltration has traditionally used polymer-based membranes, but there is growing interest in the use of ceramic membranes for produced water treatment, due to their longer operational life and higher throughput capabilities. BENEFITS AND LIMITATIONS For produced water applications, ultrafiltration will mainly be used as a pretreatment if downstream desalination is required. Traditionally, ultrafiltration has been accomplished using a polymeric material such as PolyAcryloNitrile (PAN) or PolyVinyliDene Fluoride (PVDF). These materials can be degraded by harsh organic contaminants and irreversibly fouled by oil and grease. Ceramic membranes have extremely high thermal, chemical and mechanical stability, and therefore can withstand strong cleaning chemicals and elevated temperatures, and will not be susceptible to breakage of the material. These characteristics have significant implications for maintenance of the membrane process under harsh environmental and operational conditions. For example, ceramic membranes can be cleaned using steam and/or high- and low-pH chemicals. Ceramic membranes are also resistant to oxidants such as ozone and chlorine, which would cause degradation of polymeric membranes.2 These membranes are also resistant to organic acids that may be present in feed water sources. All of these benefits lead to a significantly longer operational life as compared to polymeric membranes. Ceramic membranes are also capable of achieving flux rates between 100 gfd (gallons of fluid passing through a square foot of membrane surface per day) and 300 gfd—about 3–10 times the maximum flux rates of polymeric membranes. Ceramic membranes have capital cost that is roughly four to five times that of a polymeric membrane per square foot of material, Table 1. Thus, the capital cost may be higher for ceramic membranes, but because of the numerous advantages of ceramic membranes mentioned above, the total cost over the life of a producing well or field may be lower for ceramic membranes than for polymeric membranes. Another potential limitation to the use of ceramic membranes is that, at present, there is little experience in the US using ceramic membranes for oilfield water treatment applications.
CERAMIC MEMBRANE CHARACTERIZATION Ceramic membranes are monolithic structures made from oxides, nitrides or carbides of metals such as aluminum, titanium and zirconium.3 Typically, a tubular configuration is used with an inside-out flow path, in which the feed water flows inside the membrane channels and permeates through the support structure to the outside of the module. These membranes are typically comprised of at least two layers, a support layer and a separating layer, Fig. 1.
Permeance and membrane resistance are two parameters that are commonly used to describe membrane materials. Permeance is a measure to describe the normalized productivity of membranes and is most often reported by measuring the flux of deionized water through the membrane at a given pressure. Ceramic membranes have a much higher permeance than polymeric membranes and can therefore produce more clean water than polymeric membranes can at the same pressure, or the same volume of water at a lower pressure—therefore using less energy. Ceramic membranes also have a lower membrane resistance (resistance to flow), Table 2.
CERAMIC MEMBRANE OPERATION Ceramic membranes are capable of removing particulates, organic matter, oil and grease, and metal oxides. Ceramic membranes alone cannot remove dissolved ions and dissolved organics. Pre-coagulation—injection of a chemical coagulant upstream of the membrane—improves removal efficiencies of dissolved organic carbon and smaller particulates. As with typical ultrafiltration or microfiltration, a strainer or cartridge filter is necessary as pretreatment for ceramic membranes. There are two modes of operation in which ceramic membranes can be utilized: cross-flow and dead end. In dead-end filtration mode, all of the water that is fed to the membrane passes through the membrane as filtrate, or product water. In cross-flow mode, some of the feed water continues through the module untreated. Cross-flow mode can minimize membrane fouling but also generates a waste stream for disposal. Dead-end filtration requires more frequent backwashes and may cause more irreversible fouling of the membrane, or fouling that cannot be removed by backwash or cleaning. Regardless of the mode of operation, ceramic membranes require frequent backwashes, and the backwash waste will require disposal. RESEARCH AND COMMERCIAL USE Numerous studies have been conducted on using ceramic membranes to treat oil containing wastewater and produced water.4–8 These studies have shown that ceramic membranes perform better than polymeric membranes on oil-containing waters. Though many companies offer ceramic membrane products of various sizes, channel geometries and materials of construction (Table 3), commercial use in the oil and gas industry is still very limited. In one example, ceramic membranes are employed as part of a large, multi-process treatment train at the Wellington Water Works to treat oilfield produced water.9
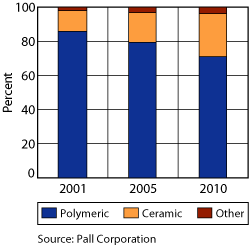
Ceramic membranes are a more established technology in other industries, such as biopharmaceuticals, food and beverage, and industrial wastewater. In these applications, the feed solutions contain much higher concentrations of suspended solids than oilfield produced waters contain. Thus, these applications require much more conservative modes of operation, meaning higher cross-flow velocities and more frequent backwashes and cleanings. In produced water applications, ceramic membranes can be operated with less cross-flow or possibly dead-ended with less-frequent backwashes and cleanings. Most ceramic membrane manufacturers also make stainless steel housings for their membranes, which are not necessary or optimal for treatment of produced water and are more costly than other materials, such as plastics. As more research and pilot studies are conducted using ceramic membranes for produced water treatment, understanding of the optimal operating conditions and materials will improve. Thus, their capital and operating costs will continue to decrease as they become more widely used, Fig. 2.
CONCLUSIONS Ceramic membranes may be a viable treatment technology for some produced water applications. Ceramic membranes will most likely be needed as a pretreatment technology if desalination is required. There are many different manufacturers that supply a wide range of materials and membrane sizes to meet the needs of specific applications. Ceramic membranes offer advantages over polymeric membranes, such as increased chemical, mechanical and thermal stability and higher productivity. While the capital costs of ceramic membranes are presently higher than polymeric, the higher productivity and longer lifespan indicate that ceramic membranes may have an overall lower lifecycle cost than polymeric membranes. Furthermore, as ceramic membranes become a more widely used technology in water treatment applications, the cost of these products will continue to decrease. LITERATURE CITED 1 Benko, K. L. and J. E. Drewes, “Produced water in the western United States: Geographical distribution, occurrence, and composition,” Environmental Engineering Science, 25, No. 2, 2008, pp. 239–246.
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
- Shale technology: Bayesian variable pressure decline-curve analysis for shale gas wells (March 2024)
- What's new in production (February 2024)
- Prices and governmental policies combine to stymie Canadian upstream growth (February 2024)
- U.S. operators reduce activity as crude prices plunge (February 2024)
- U.S. producing gas wells increase despite low prices (February 2024)
- U.S. drilling: More of the same expected (February 2024)