Injecting surfactants into the water stream allows reliable monitoring of sub-ppm oil concentrations.
Dale F. Brost and Joshua Silveira, Turner Designs Hydrocarbon Instruments, Inc.
Surfactants have been facilitating oil-in-water measurements by fluorescence in California steamfloods for more than 10 years. Two main benefits have been realized. First, the selective amplification of dispersed oil fluorescence has made it possible to reliably monitor sub-ppm concentrations of dispersed oil in the presence of highly fluorescent Water-Soluble Organics (WSOs). Second, the cleaning action provided by continuous surfactant injection makes it practical to use flow-cell instruments in place of falling-stream instruments. The reduced water flow required by a flow cell reduces surfactant cost significantly.
Recent field studies suggest that a surfactant modulation technique could allow one online fluorometer to monitor both dispersed oil and WSOs. This article proposes such a technique.
INTRODUCTION
Aromatic fractions of dispersed oil and WSOs in produced water can be stimulated to emit fluorescent light. Fluorescence is an extremely sensitive analytical technique, capable of monitoring most oils in produced water at concentrations less than 1 mg/L. The intensity of a fluorescence emission is proportional to the concentration of fluorescent molecules. Commercial fluorometers are available that monitor the oil content of grab samples. Others are designed to monitor oil online in a flowing produced water stream.
Surfactant molecules are bipolar, composed of a hydrophilic (water-loving) end and a lypophilic (oil-loving) end. This structure allows surfactant molecules to modify the physical properties of oil/water mixtures depending on the surfactant’s Hydrophilic-Lypophilic Balance. Surfactants with an HLB less than 10 stabilize water-in-oil emulsions. Surfactants with an HLB greater than 10 stabilize oil-in-water emulsions. Within that group, surfactants with an HLB of 12-15 act as detergents and are commonly used to remove oil from solid surfaces.
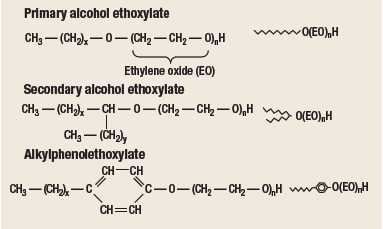
Many types of detergent surfactants are available. The non-ionic surfactants known as alcohol ethoxylates and alkylphenol ethoxylates are particularly effective for solubilizing crude oil in produced water.1,2 As illustrated by the general chemical structures shown in Fig. 1, the lypophilic ends of these surfactants contain various types of hydrocarbons. The hydrophilic ends are polymers of ethylene oxide.
 |
|
Fig. 1. Chemical structures of non-ionic surfactants. A simplified representation is shown to the right of each formula.
|
|
There are two main types of alcohol ethoxylates: primary and secondary, named for the position of the ethylene oxide groups on the hydrocarbon chain. Primary alcohol ethoxylates have a linear hydrocarbon chain with the ethylene oxide groups at the end of the molecule. Secondary alcohol ethoxylates have their ethylene oxide groups attached to various positions in the center of the hydrocarbon chain, resulting in a “two-tailed” structure. Alkylphenol ethoxylates include a benzene ring as part of their hydrocarbon chain.
TESTING SOLUBILIZATION OF CRUDE OIL
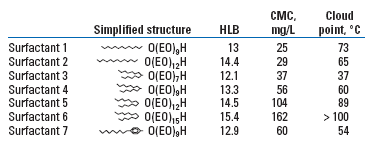
A variety of commercially available alcohol ethoxylates were studied in the lab to determine their relative ability to solubilize a range of crude oils. One alkylphenol ethoxylate was also studied. All of the surfactants selected fall into the general category of detergents, with HLB values in the 12-15 range, Table 1.
| TABLE 1. Selected non-ionic surfactants and their physical properties |
 |
|
The intensity of fluorescent light emitted by a heterogeneous mixture of oil and water is somewhat dependent on the particle size distribution of the oil droplets. For any given oil, small particles emit more fluorescent light than large particles. Since solubilization converts large oil droplets to tiny micelles, the degree of solubilization can be determined by measuring the increase in fluorescent light intensity when a non-fluorescent surfactant is added to a mixture of oil and water. The increase is easily expressed in terms of the fluorescence amplification factor, AF:

where IF, water + surfactant is the fluorescent light intensity emitted by the oil dispersed in water containing surfactant at a concentration greater than its Critical Micellar Concentration (CMC), and IF, water is the fluorescent light intensity emitted by the oil dispersed in water alone.
Crude oils from California, China and the North Sea were selected to span a range of API gravity from 13 to 27°API. NIST 1582, a standard reference oil containing a variety of large Polycyclic Aromatic Hydrocarbons (PAHs), was also selected. A 1,000-mg/L standard solution of each oil was prepared by finely dispersing 100 mg of oil in 100 ml of 99% IsoPropyl Alcohol (IPA) with the aid of an ultrasonic bath. The IPA standards were then used to prepare several sets of aqueous samples, each containing 10 mg/L of oil. Set 1 was made by adding 1 ml of each IPA standard to 99 ml of distilled water. Set 2 was made by adding 1 ml of each IPA standard to 99 ml of distilled water containing 3,000 mg/L of surfactant 1. Sets 3 to 8 were prepared similarly, with each set containing a different surfactant from Table 1.
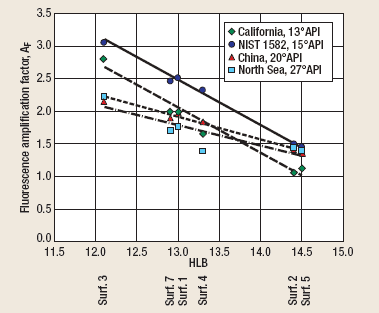
The results (Fig. 2) indicate that AF is a linear function of HLB for all the test oils, with maximum fluorescence amplification at an HLB value of 12.1. The surfactants should be expected to act somewhat differently when dissolved in produced water. A variety of factors affect solubilization (including temperature, salinity, hardness and dissolved organics) that were not included in this experiment. However, all the surfactants with HLB values of 13.3 and below should be good candidates for field application.
 |
|
Fig. 2. Fluorescence amplification factor vs. HLB.
|
|
The lines in Fig. 2 can be divided into two groups according to slope. The first group (thick lines) represents the heavy oils (California 13°API crude oil and the NIST 1582 reference oil). The second group (thin lines) represents the lighter oils (China 20°API and North Sea 27°API). The AF values for the heavy oils are significantly greater than those for the light oils at the low end of the HLB scale. This is probably due to the relatively high percentage of large PAHs in the heavy oils. These substances do not normally disperse well in pure water, but are known to be readily solubilized by detergent surfactants.3
FLUORESCENCE OF NON-IONIC SURFACTANTS
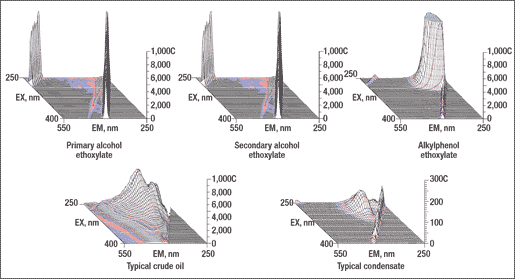
Figure 3 shows typical 3D fluorescence spectra of selected non-ionic surfactants, recorded by a fluorescence spectrometer. The surfactants were dissolved in distilled water at 200 mg/L, which is typical for monitoring produced water. Spectra for a typical crude oil and condensate are also shown. The oils were dispersed in distilled water at 10 mg/L.
The primary alcohol ethoxylate (surfactant 1 in Table 1) and the secondary alcohol ethoxylate (surfactant 3) emit no significant fluorescence. The high-intensity side-bands seen in the figure are not fluorescent emissions. Rather, they are spectral artifacts from a variety of other spectroscopic phenomena. The other primary and secondary alcohol ethoxylates in Table 1 have similar spectra. Because they emit virtually no fluorescent light, these surfactants can be added to any produced water stream without contributing to the fluorescence emitted by dispersed oil or WSOs.
The alkylphenol ethoxylate (surfactant 7), with a substituted benzene ring as part of its structure, emits very strong fluorescence at short wavelengths. The peak fluorescence intensity occurs at an excitation wavelength of 270 nm and an emission wavelength of 300 nm. In fact, at a concentration of 200 mg/L, the fluorescence at these wavelengths is so strong that it saturates the fluorometer’s detector. However, the fluorescence band is fairly narrow, and the fluorescence intensity drops off to an insignificant level at excitation wavelengths greater than 325 nm.
It is evident that the alkyphenol ethoxylates cannot be used without interference while monitoring condensates at short wavelengths. The spectral overlap between these two substances is too severe. However, the alkyphenol ethoxylates can be considered for water systems containing crude oils that emit adequate fluorescence at longer wavelengths.
FIELD STUDIES
All of the surfactants listed in Table 1 are supplied by their manufacturers in 100% active form. They are either viscous liquids or white semi-solids at room temperature and do not readily mix with water. Because of this, they must be diluted in water, typically to a working concentration of 30%, before they can be easily pumped into produced water in the field. Also, if ambient temperatures are expected to approach or drop below 0°C, it may be necessary to heat the 30% active surfactant blend, or to add a freezing-point depressant (e.g., methanol). Depending on the produced water chemistry, it may also be beneficial to add acetic or citric acid to the blend to inhibit the precipitation of iron solids, or to help release oil that is already trapped by iron solids.
Two surfactant formulations that are stable to -20°C are:
- Formulation A: 30% surfactant (composed of surfactants 1, 2, 3 and 7), 25% acetic acid and 45% distilled water
- Formulation B: 30% surfactant (same composition), 19% methanol and 51% distilled water.
As discussed above, the primary and secondary alcohol ethoxylates emit no fluorescent light. To maintain this desirable quality in the final blend, care must be taken to avoid contamination by fluorescent substances from other products, such as aromatic solvents, corrosion inhibitors and emulsion breakers. If the surfactant blend contributes significantly to the produced water’s fluorescence, the interference must be subtracted during instrument calibration, and the surfactant concentration must be carefully controlled.
Field installations. In practice, surfactants are added to a side stream of water as it flows from the process to the instrument. To ensure adequate solubilization of dispersed oil, the surfactant is injected at a concentration well in excess of its CMC.
A typical installation requires a sample pump to move water through the instrument. Surfactant is injected as far upstream of the instrument as possible to prevent oil and oily solids from sticking to the tubing walls. This helps ensure that all the dispersed oil entering the tubing actually reaches the instrument. A sample port must be installed upstream of the surfactant injection port to allow collection of a surfactant-free water sample. This is necessary if grab samples are to be analyzed by a solvent-extraction method to calibrate (or validate) the online instrument. If dispersed oil has been solubilized by the surfactant, it will not be quantitatively extracted into the solvent, and the test will give false low results.
A variety of pumps can be used, such as centrifugal and diaphragm. Care must be taken, especially with diaphragm pumps, to prevent cavitation by ensuring that the pump suction is flooded. Cavitation can cause the surfactant to generate opaque foam that may interfere with the fluorescence measurement.
A length of tubing is required between the surfactant injection port and the instrument to allow the surfactant time to solubilize the dispersed oil. The amount of “soak” time required depends on surfactant type and concentration, water temperature, oil loading and particle size distribution, among other factors. Experience with steamflood water streams, where water temperature is usually greater than 60°C, has shown that a minimum of 30 sec. is recommended for good solubilization at the flotation cell outlet. A sample cooler is recommended whenever the water temperature is greater than the surfactant’s cloud point.
Fouling prevention by continuous injection. As mentioned previously, surfactants can be used to reduce fouling by dispersed oil and oily suspended solids. The authors recently installed an oil-in-water monitor at the outlet of a flotation cell at a California waterflood project, with 32°C water and dispersed oil gravity of 20°API. Water samples taken at the site were heavily loaded with iron sulfide particles that were glued together by dispersed oil. Also, samples collected in a pressure bomb (with care to exclude air contamination), turned black almost immediately, indicating that additional iron sulfide was precipitating in the system.
The monitor contains a falling-stream water sampler that is normally resistant to fouling because its non-contact design never allows the water stream to touch an optical surface. However, the water flowrate through the instrument was shutting off completely in less than a day due to the accumulation of an oily iron sulfide paste in the upstream tubing. A trip to a local chemical company resulted in a drum of Formulation A (see above) blended with an alkylphenol ethoxylate (nonylphenol with nine ethylene oxide groups, similar to surfactant 7). The surfactant blend was injected into the water stream flowing to the instrument at a rate that yielded 235 mg/L of active surfactant. The untreated samples turned black in less than 30 sec. The surfactant-treated samples remained clear for hours, indicating that the surfactant blend effectively prevented iron sulfide precipitation.
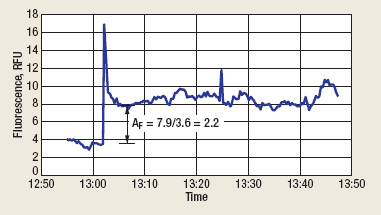
The instrument was configured with excitation and emission wavelengths that provided good sensitivity and linear ranges for the oil, but were long enough to eliminate interference from the alkylphenol ethoxylate, Fig. 3. As shown in Fig. 4, the dispersed oil fluorescence amplification factor (AF) was 2.2, which is in substantial agreement with the lab results discussed above for 20°API oil and surfactant 7 (also an alkylphenol ethoxylate). It should be noted that the fluorescence of the WSOs at this site was effectively zero (0.09 Relative Fluorescence Units, RFU), making it possible to compute AF directly from the values in the trend plot. It should also be noted that a fluorescence spike usually occurs when the surfactant first reaches the instrument. This is a result of the surfactant’s detergent action, releasing oil that was coating the inside surfaces of the tubing before surfactant injection began.
 |
|
Fig. 3. Fluorescence spectra of surfactants (200 mg/L) and oils (10 mg/L).
|
|
 |
|
Fig. 4. Amplification of dispersed oil fluorescence.
|
|
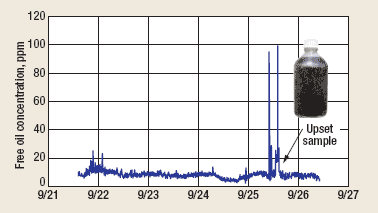
The instrument was calibrated with the surfactant pump running, using grab samples taken upstream of the surfactant injection port. The instrument was left operating without maintenance for the next 6 days, with no sign of fouling. Figure 5 shows a trend plot of the oil concentration data logged during this period. Note the upset on Sept. 25. A water sample taken from the instrument’s effluent during the upset (inset) contained a high level of large iron sulfide particles. When the solids were isolated and extracted with solvent (perchloroethylene), the extract was nearly colorless, indicating low oil content. This shows that the surfactant effectively removed the oil that was normally attached to the iron sulfide, which prevented the iron sulfide from sticking to the tubing walls. After the upset, which lasted several hours, the instrument remained fully functional without a trace of fouling. Visual inspection of the tubing interior on Sept. 26 revealed only shiny metal surfaces with no iron sulfide deposits from the injection point to the instrument’s drain.
 |
|
Fig. 5. Surfactant-assisted oil concentration trend.
|
|
Steamflood applications. Steamflood operations require special attention to water quality. Dispersed oil concentration must be kept very low, typically less than 2 mg/L, to control oil fouling in water softeners and steam generators. Water plants that use reverse osmosis membranes are extremely susceptible to fouling by dispersed oil. Membranes can also be fouled if the concentration of WSOs (e.g. napthenic acids) in the reject water increases beyond the solubility limit.
Steamflood produced water contains heavy crude oil (~13°API). Dispersed oil droplets in a flowing water stream typically have diameters from 1 to 200 µm (dependent on temperature, shear forces, emulsion breaker performance, etc.). Oil can also be associated with suspended solids.
Surfactants can assist online fluorescence monitors in a number of ways. They can dramatically increase the ability to detect ultra-low concentrations of dispersed oil in the presence of highly fluorescent WSOs. They can also assist with the transport of dispersed oil from the process to the instrument, by preventing oil from adsorbing onto the interior surfaces of metal tubing. In addition, surfactants can release oil droplets from suspended solids, making them available for measurement. They can also make it possible to monitor both dispersed oil and WSOs with one online instrument.
The total fluorescence emitted by produced water is the sum of the fluorescence emitted by the dispersed oil and the fluorescence emitted by the WSOs: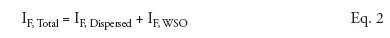
with all units in RFU. Steamflood produced water contains high concentrations of fluorescent WSOs that make IF, WSO a large part of IF, Total. In some cases, IF, WSO is so large that IF, Dispersed becomes difficult to detect. This is especially true at the ultra-low dispersed oil concentrations encountered in steamflood systems (< 2 mg/L).
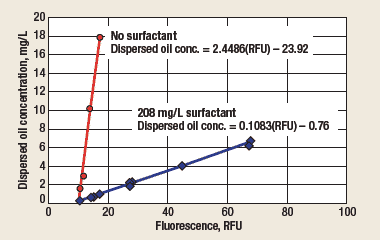
Dispersed oil fluorescence amplification. Figure 6 shows typical calibration curves for a falling-stream online fluorometer installed at the effluent of a flotation cell. To illustrate the surfactant-induced amplification of dispersed oil fluorescence, calibration curves were determined with and without surfactant injection. The surfactant was surfactant 1, blended as described above for Formulation A. Calibration was performed by correlating fluorescence intensity readings to the dispersed oil content of grab samples collected upstream of the surfactant injection point. The grab samples were analyzed by a hexane extraction method using a handheld oil-in-water analyzer. The method was made selective for dispersed oil by performing the extraction at neutral pH. The dispersed oil concentration was varied over a range by briefly adjusting the operating parameters of the flotation cell.
 |
|
Fig. 6. Dispersed oil calibrations with and without surfactant.
|
|
The calibration curves converge at a fluorescence intensity of 9.5 RFU when extrapolated to a dispersed oil concentration of zero. This is the fluorescence of the WSOs, which remains essentially constant with time. The slope of the “no surfactant” curve is 2.4486 mg/L dispersed oil per RFU. Since the instrument’s rated stability is ±1 RFU over a 6-month period, the maximum long-term drift could be as much as ±2.4 mg/L of dispersed oil. This is not adequate to monitor dispersed oil in the 0-2-mg/L range. However, with surfactant present at 208 mg/L, the fluorescence emitted by the dispersed oil was amplified by a factor of 22.6, resulting in a calibration slope of 0.1083 mg/L dispersed oil per RFU. This predicts a maximum long-term drift of only ±0.1 mg/L dispersed oil.
Lab results summarized in Fig. 2 show that surfactant 3, a secondary alcohol ethoxylate, should also efficiently solubilize dispersed heavy oil and amplify dispersed oil fluorescence. A field test was conducted with a 30% solution of surfactant 3 in distilled water, using the same instrument and procedure described above. When applied at 200 mg/L, surfactant 3 amplified the dispersed oil fluorescence by a factor of 9.8, resulting in a dispersed oil calibration slope of 0.2504 mg/L dispersed oil per RFU. However, the temperature of the water stream was 62°C, which is greater than the cloud point of the surfactant, 99°C. Inspection of the surfactant-treated water stream revealed significant turbidity. When the water stream’s temperature was reduced to 35°C, the turbidity disappeared and the fluorescence amplification factor increased to 13.7.
INSTRUMENT SELECTION
In California steamflood operations, surfactants have been used to enhance the performance of online fluorometers since 1993. The early instruments used falling-stream measurement cells,1 but recently these fluorometers were replaced with a flow cell instrument, based on a number of considerations:
1. Extensive field trials, conducted over 6 months, showed that continuous surfactant injection was effective in keeping the flow cell free of oil and oil-coated suspended solids.
2. The water rate through the flow cell can be as low as 1 L/min., significantly lower than the 8 L/min. required by the falling-stream cells. This greatly reduces the surfactant rate required to achieve an effective surfactant concentration. Also, the reduced water rate made it possible to use smaller, less expensive sample coolers to reduce the water temperature below the surfactant’s cloud point.
3. The flow-cell instrument includes a photometric module that continuously monitors the cleanliness of the flow cell and relay contacts that can be used to alert operators if cleaning should be required.
4. The flow-cell instrument can be configured with an automatic cleaning system that can circulate cleaning fluid through the flow cell if its transmission efficiency decreases to a point that would affect the oil measurement.
5. The flow cell has a pressure rating of 250 psi. This allows water that has passed through the flow cell to be returned to the process or discharged to a pressurized drain. Falling-stream instruments require an atmospheric pressure drain.
The flow cell is currently monitoring 17 steamflood water streams in California. The methodology and results discussed in the remainder of this paper are specific to this instrument.
DISPERSED OIL CALIBRATION
A flow-cell oil-in-water monitor using an online fluorometer can be calibrated for heavy oil service as follows:
1. Zero the instrument by injecting a surfactant-free produced water sample into the flow cell through a syringe filter (glass fiber prefilter and 0.2-µm cellulose acetate membrane).
2. Determine the calibration slope by correlating the fluorescence measured by the instrument to the dispersed oil concentrations of grab samples.
Figure 7 shows dispersed oil monitoring data from a California steamflood. Trends from two flow-cell monitors are shown. Instrument 1 was installed at the effluent of a flotation cell. Instrument 2 was installed just downstream, at the inlet of a walnut-shell filter. A 5,000-bbl pump suction tank was located between the flotation cell and the filter.
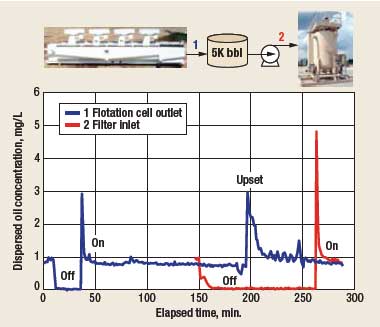 |
|
Fig. 7. Fluorescence monitoring trends.
|
|
The trend from instrument 1 begins normally, with the surfactant pump running and the instrument correctly reporting dispersed oil concentration. The surfactant pump was then turned off, which caused the dispersed oil concentration to be reported as zero, showing that the fluorescence emitted by dispersed oil without the surfactant dropped to an insignificant level.
After about 35 min. had elapsed, the surfactant was turned back on. As soon as the newly injected surfactant arrived at the instrument, the dispersed oil response spiked. As explained above, this is due to the surfactant’s ability to wash oil from the tubing’s inside surfaces on its way to the instrument. The spike soon disappeared, and the dispersed oil readings returned to normal. At 150 min., the surfactant pump at instrument 2 was shut off, causing its dispersed oil reading to fall to zero. At 180 min., water flow through the plant was shut off. However, water from the stagnant process continued to flow to both instruments. When the process water flow was restored, dispersed oil concentration monitored by instrument 1 increased due to a surge of water through the flotation cell. Instrument 1 continued to track the upset, which eventually dissipated. However, since the surfactant for instrument 2 was shut off, it did not detect the upset, even though it was installed a short distance downstream. This is further evidence that the instrument can be rendered completely insensitive to dispersed oil by turning off the surfactant.
This phenomenon was further investigated over a 7-week period, using a flow-cell monitor installed at the inlet to a flotation cell. Two measurements of WSO fluorescence were taken at regular intervals. The first was a measurement of the fluorescence detected by the instrument after the surfactant had been turned off for 1 hr (“surfactant-off” method). The second measurement was made on a filtered, surfactant-free sample (“filter” method).
Though the dispersed oil concentration varied significantly during the test period, the WSO concentration was found to remain nearly constant. Also, both WSO fluorescence measurements changed very little and had essentially equal mean values, demonstrating that both methods give the same result and are independent of dispersed oil concentration. Therefore, when performing a dispersed oil calibration, the instrument can be zeroed by either method.
In the 7-week test, a mean WSO concentration of 31.7 mg/L gave a mean fluorescence of 10.4 RFU by the “surfactant-off” method. Distilled water, containing negligible WSOs, emitted no detectable fluorescence. These observations suggest that a flow-cell oil-in-water monitor can also be used to monitor WSO concentration simply by turning off the surfactant.
The appropriate calibration function is:

where CWSO is the WSO concentration (mg/L), IF, WSO is the fluorescence intensity emitted by the WSOs (RFU), and kWSO is the slope of the WSO calibration curve (mg/L per RFU). IF, WSO can be determined by either the “filter” or the “surfactant-off” method to solve for kWSO.
SURFACTANT MODULATION METHOD
The results reported above indicate that surfactant modulation (alternately turning the surfactant pump on and off) can be used to separate the fluorescence emitted by dispersed oil from that emitted by WSOs. This allows one instrument to monitor both substances. When the surfactant pump is on, the instrument measures the total fluorescence emitted by the water stream, IF, Total. When the surfactant is off, the dispersed oil fluorescence becomes insignificant and only the WSO fluorescence, IF, WSO, is detected. The dispersed oil fluorescence, IF, Dispersed, can then be determined by subtracting IF, WSO from IF, Total.
A surfactant modulation method is proposed here:
1. Calibrate instrument and store calibration slopes.
2. Turn surfactant pump off and wait for fluorescence to stabilize.
3. Measure and store IF, WSO.
4. Compute and store CWSO.
5. Turn surfactant pump on and wait for fluorescence to stabilize.
6. Measure IF, Total and compute IF, Dispersed, CDispersed and CTotal.
7. If it is time to update WSO values, go to step 2. Otherwise go to step 6.
Step 7 requires the user to decide how often to update WSO measurements and calculations. Normally, WSO concentration changes very slowly, depending on the residence time of process vessels upstream of the monitoring point. WSO molecules are completely dissolved in the process water and do not pass through the process in slugs (as is common for dispersed oil). This means that WSO measurements can be taken infrequently, updating every few hours. It is important to select a WSO update interval as long as possible to avoid missing dispersed oil upsets while the surfactant is turned off. 
LITERATURE CITED
1 Brost, D., “Water quality monitoring at the Kern River Field,” presented at the 14th Annual Produced Water Society Seminar, Houston, Jan. 20-22, 2004.
2 Morrow, L., “Fluorescence method of quantifying hydrocarbons, including crude oil dispersed in water,” US Patent 5381002, 1995.
3 Swe, M. M. et al., “Solubilization of selected polycyclic aromatic compounds by nonionic surfactants,” Journal of Surfactants and Detergents, 9, No. 3, September 2006, pp. 237-244.
4 Brost, D. and G. Bartman, “Updates on fluorescence monitoring of produced water,” presented at the 16th Annual Produced Water Society Seminar, Houston, Jan. 25-27, 2006.
|
THE AUTHORS
|
|
|
Dale F. Brost is the Director of Technology Development for Turner Designs Hydrocarbon Instruments, Inc., in Fresno, California. He received his BS degree in chemistry from Loyola University of Los Angeles in 1973, and his PhD in chemistry from Baylor University in 1978. Dr. Brost worked for Texaco for 22 years on oilfield chemistry-related research projects and field assignments. Since 1989, he has specialized in water quality monitoring instrumentation.
|
|
|
|
Joshua Silveira is a first-year PhD candidate in analytical chemistry at Texas A&M University. He earned a BS degree in chemistry from California State University, Fresno in 2007. During that time, he worked as a chemical technician for Turner Designs Hydrocarbon Instruments, Inc., where he developed spectroscopic methods and instrumentation for oil-in-water analysis.
|
|
|












