Two field studies demonstrate that a new anti-agglomerate low-dosage hydrate inhibitor does not affect oil/water quality.
Rama Alapati, Jeremy Lee and David Beard, Champion Technologies
First-generation Anti-Agglomerate Low Dosage Hydrate Inhibitors (AA-LDHI) are known to perform at low watercuts of less than 40%, but caused poor produced oil/water quality. The second-generation new AA-LDHI chemistry is predominantly oil soluble and has been proven to prevent hydrate blockages in both wet-tree and dry-tree applications. Recently, a new chemistry was successful in field applications with watercuts up to 80%.
A major benefit of this new chemistry is that produced water quality is improved in field applications. Its oil-soluble nature makes produced water less toxic and can reduce chemical costs for produced water polishing. This article explains the new chemistry and its benefits.
Hydrate management is a critical aspect of deepwater oil and gas production facilities, since wells and flowlines are susceptible to hydrate blockage, if natural gas and water are present at elevated pressures and low temperatures. The temperature below which hydrates can form increases with increasing pressure and can be as high as 86°F.1 Once formed, the blockage can take from days to months to dissociate, resulting in decreased revenue. Remediation of a hydrate plug poses significant risk to personnel safety and comes with a potentially negative environmental impact, since dissociating a hydrate plug can cause pipeline/flowline rupture.
Flow assurance requirements are often the design basis for deepwater field developments, and can be thermal and/or chemical. Typical methods for hydrate control include pressure reduction, changing the system temperature by insulating equipment, circulating hot oil prior to startup/re-start and injection of anti-agglomerate, kinetic or thermodynamic inhibitors.
The conventional chemical method is to use thermodynamic inhibitors, like methanol and glycol. This is a proven technology; however, the logistics of transportation and storage of large inhibitor volumes have a significant impact on Opex and Capex.2 Also, the considerations for umbilical and pump sizes, topsides space and larger equipment weight have led to alternatives.2 There is also the limitation set by refineries on allowable methanol concentrations in export oil or condensate. First- and second-generation AA-LDHIs have shown no adverse effect on refining processes or refinery products.3 Hence, the industry has incentives to use the alternatives, which are the low-dosage hydrate inhibitors.2
LDHIs are classified as either Kinetic Hydrate Inhibitors (KHI) or AA-LDHI. KHIs are polymers that delay hydrate nucleation and crystallization in flowlines or wells. They are effective at concentrations of 0.5-3.0%, which is significantly lower than the thermodynamic inhibitors. KHIs are not suitable for all hydrate conditions (especially in the deep and ultra-deep GOM) because they only handle low to moderate subcooling of 10-25°F. KHIs can only provide protection for hours, which poses a problem when systems are shut down for longer periods. AA-LDHIs are effective at low dosage rates of 0.5-1.5 wt%, based on the water phase, and they retain effectiveness longer than KHIs.
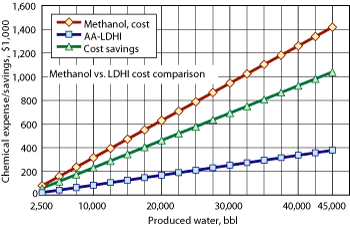
First-generation AA-LDHIs are known to perform at watercuts up to 40%, and producers have considered this in their design criteria for new deepwater developments. Using methanol for watercuts over 40% is less economical than an AA-LDHI that works at these high watercuts, Fig. 1.
 |
|
Fig. 1. Protecting 45,000 bbl of water production with AA-LDHI could save a producer over $1 million, compared to methanol.
|
|
The values were calculated using treatment rates of 30 vol% for methanol and 1 vol% for the AA-LDHI with $2.50/gal for methanol and $20/gal for AA-LDHI. The cost savings compare only chemical costs.
To treat 45,000 bbl of produced water with methanol at 30 vol% requires 13,500 bbl. To treat that same volume of produced water with an AA-LDHI at 1 vol% requires only 450 bbl. The 13,050-bbl difference, if crude oil, would be worth over $1 million at $90/bbl.
Some of the industry’s first-generation AA-LDHIs required a minimum of 1.5% salinity in the produced water to perform,2 which rules out using them during the early production, as the water produced could be condensed water. Some first-generation AA-LDHIs are completely water soluble or partially oil soluble and are known to cause oil/water separation issues. This could also lead to increased chemical treating costs. New deepwater developments in the GOM are requiring less than 10 ppm overboard oil and grease, which is difficult to achieve with the first-generation, watercut limited AA-LDHI. Their water-soluble nature has another negative effect-toxicity. In general, these are highly toxic quaternary salts with low bio-degradability and high bioaccumulation.
It is only a matter of time before more stringent regulations are implemented worldwide. Rigorous environmental regulations are the sole reason for not using AA-LDHI in many parts of the world. By being completely oil soluble, AA-LDHI will partition into the oil phase and the produced water will not contain any environmentally sensitive ingredients. The best solution would be to use an AA-LDHI chemistry that is completely oil-soluble, works at high watercuts and does not cause any oil/water separation issues.
Two years ago, a new AA-LDHI chemistry was introduced that provided a step change in performance at watercuts up to 80%, and for subcooling to 30°F.
WET-TREE APPLICATION
The wet-tree application is a subsea tieback that is a three-mile long, 4-in.-diameter uninsulated flowline, producing from about 2,500 ft of water depth. AA-LDHI is injected at the wellhead on a continuous basis to protect against hydrate formation. The watercut at the beginning of the treatment was 30% with production rates at 2 MMcfd gas, 1,300 bopd and 500 bwpd. The rates for the 60%-watercut production are 725 Mcfd gas, 361 bopd and 520 bwpd. The treatment rate is 0.5 vol% based on water volume. Subcooling is 25-30°F. The hydrate curve illustrates the hydrate formation region for the system, Fig. 2.
 |
|
Fig. 2. This curve illustrates the hydrate formation region for the AA-LDHI system at 0.5 vol% with subcooling to 25-30°F.
|
|
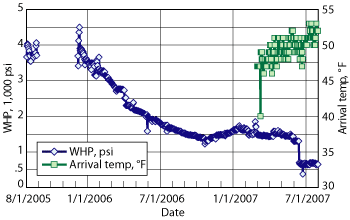
The wellhead pressure and fluid arrival temperature are plotted in Fig. 3. The graph illustrates the well parameters that can be used in conjunction with the hydrate curve in Fig. 2 to verify the wet-tree well/flowlines’ operation inside the hydrate formation region. The application of the new AA-LDHI chemistry for continuous injection and shut-in/re-starts operated successfully through maximum shut-in tubing pressures of 2,500 psi to 3,500 psi.
 |
|
Fig. 3. Wellhead pressure and fluid arrival temperature along with Fig. 2 verifies hydrate inhibition with the new chemisty.
|
|
DRY-TREE APPLICATION
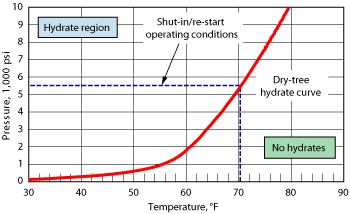
The drytree is in 4,300-ft water depth and produces 32.5 MMcfd of gas, 2,071 bopd and 9,028 bwpd. The watercut is at 80%, and water produced makes up 82% of the total production on the platform. The API gravity for the oil is about 30°API at 60°F. AA-LDHI is injected downhole just above the SCSSV during shut-in and re-starts. The treatment rate is 1.3 vol% based on the water volume. The well is set up with 117⁄8-in. casing and 5½-in. tubing with subcooling at 30°F and a shut-in tubing pressure of 4,500-5,500 psi. The hydrate curve, Fig. 4, shows the hydrate formation region for the system.
 |
|
Fig. 4. This curve shows the hydrate formation region for the AA-LDHI at 1.3 vol% with subcooling at 30°F.
|
|
The hydrate curve shows that during shut-in and re-starts the system will be operating inside the hydrate formation region and that a hydrate inhibitor will be needed. The parameters that determine the hydrate risk in a shut-in well are the watercut and the Gas-to-Oil Ratio (GOR). These two parameters determine the height of the fluid level in the tubing. High watercut and low GOR signify that the liquid level may be at a height above the mudline and as a result, the oil/water mixture is subject to hydrating, due to the colder temperatures and heat transfer experienced there. Cool-down studies have indicated that the cool-down time to hydrate temperature at the mudline is 2-3 hr and does not change much with well geometry, insulation, GOR, watercut or production rates prior to shut-in.4
Shut-in operating procedures ensure hydrate inhibition. For shut-ins, AA-LDHI is injected into the well until the treated fluid displaces the tubing. For re-starts, AA-LDHI injection is initiated and continues until arrival temperature increases above the hydrate formation temperature.
During re-starts, the well is produced through the test separator. Oil is directed to the crude oil treater for further processing, and the water is directed to a 500-bbl cleanup tank for additional retention time. Water from the cleanup tank is pumped back to the system through the produced water degasser gradually over time to minimize system upsets.
A field trial was performed in March 2007 to compare the new chemistry with a first-generation AA-LDHI. The first-generation inhibitor was causing severe water quality issues. It increased water clarifier usage during flowback and still resulted in poor water quality. Normal recovery took several days and increased chemical and labor costs.
The old chemistry was tested first followed by the new AA-LDHI chemistry. The field trials were conducted over a three-day period.
Day 1. The well to be tested was lined up to the test separator, and the system was allowed to stabilize with the well in test. First-generation LDHI injection was begun and ramped up to 2.5 gal/min. (0.5 vol%). After a pre-determined fluid volume passed through the test separator, the oil dump line was directed to the oil treater and the water dump line was directed to the cleanup tank to minimize system upsets. Once the treated fluids were flowing through the test separator, oil and water samples were taken from the separator dump lines to measure the Total Oil and Grease (TOG) in the water and the percent BS&W in the oil. Values of TOG and BS&W percent were collected for two separate produced volumes. BS&W was monitored for problems, and the water in the cleanup tank was held (70-80% full) until it could be pumped back into the system.
Day 2. A flush procedure displaced the first-generation AA-LDHI from the injection line and filled the line with the new AA-LDHI chemistry for the second half of the field trial.
Day 3. The same procedures were carried out to test the new AA-LDHI chemistry.
Using the new chemistry, flowback took hours to complete versus days with the older chemistry. Water quality improved and no system upsets occurred during a flowback.
NEW CHEMISTRY
The new AA-LDHI chemistry is a proprietary composition of quaternary salts used for inhibiting, mitigating and/or delaying formation of hydrates or agglomerates.
Its oil-soluble nature is critical to performance and makes it more environmentally friendly. Once injected into the system, the chemistry coats small hydrate crystals as they form and prevents their agglomeration, thereby protecting the system from plugging. The hydrate slurry formed will disperse into the hydrocarbon phase and pass through the system until a temperature rise melts the slurry and hydrate components dissociate.
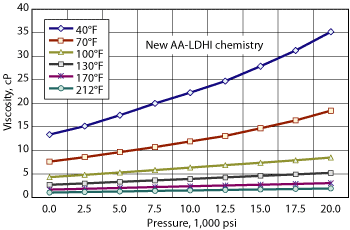
The viscosity of the new chemistry under high pressure and varying temperatures is critical for applications involving pumping over long distances and/or through small-diameter tubing, as is normal for deepwater/ultra-deepwater fields, Fig. 5.
 |
|
Fig. 5. The new chemistry’s viscosity under high pressure and varying temperatures is critical for deepwater applications.
|
|
It is extremely important that the viscosity be low enough to allow for treatment at the recommended activity dosage. In some instances, products would have to be diluted to meet the viscosity specifications for pumping. This can increase the volume needed, which increases equipment sizes. Some first-generation chemistries have viscosities in the 70-80-cP range at 10,000 psi and 40°F, which creates problems over long distances.
Temperature stability of the chemistry is important because the material will go through a wide range of temperature variations during transportation, storage and injection through the umbilicals. If the inhibitor is to be injected downhole, it will go through a hot, cold, then hot cycle as it passes from topsides to the umbilical on the seafloor and then to the wellbore where temperatures can be above 200°F.
Materials for subsea injection must meet a NAS 1638 specification rating of Class 8 or better. The new chemistry was put through a rigorous temperature stability and cleanliness test loop to determine stability. The chemical was cycled for 14 days while the pressure drops across the different sections of the loop were monitored. Consistent pressure drops validated that there was no thermal breakdown of the components or plugging of lines and filters during extreme temperature changes.
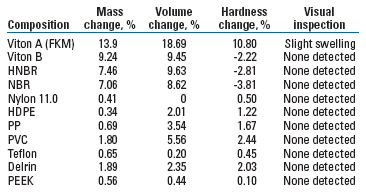
Materials compatibility. Interactions with common elastomers, thermoplastics and metals that AA-LDHI would come into contact with were analyzed for compatibility. Elastomers and thermoplastics were submerged in the new chemistry and placed in ovens at the specified temperature for 28 days. After heat soaking, measurements were taken to calculate the change in the materials’ physical characteristics: mass, volume and hardness. Visual inspection included checks for swelling, blistering and cracking, Table 1.
| TABLE 1. New AA-LDHI chemistry compatibility with elastomers and thermoplastics at 140°F |
 |
|
For metals, the same procedure was followed except that they were tested for one week, which allows for corrosion rate measurements. Corrosion rates were less than 0.02 mpy at 140°F. Materials were tested at varying temperatures according to the field parameters. In general, the new chemistry is compatible with any material that is compatible with methanol.
Toxicity. Seven-day static-renewal toxicity tests were conducted by a third-party lab to determine toxicity of the new chemistry to both M. beryllina larvae and M. bahia juveniles. The tests were performed according to the requirements of the Western Gulf of Mexico OCS General Permit and/or Methods 1006 and Methods 1007, respectively. The new chemistry was found to be in compliance for both M. beryllina larvae and M. bahia juveniles, if the critical dilution was less than or equal to 2% produced water.
FIELD RESULTS
The following are from the two field case studies that applied the new AA-LDHI chemistry.
Wet tree. The switch from methanol to the continuous injection of the new chemistry was completed without any issues, and the new chemistry continues to prevent the formation of hydrate blockages for the subsea application. The capability of the new chemistry to prevent hydrate plugs during shut-ins has also been confirmed by the numerous shut-in and restarts performed over the last two years. The shut-in durations ranged from one to three days.
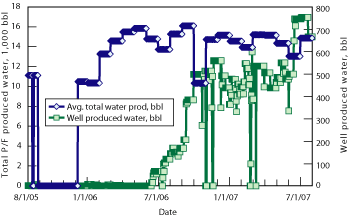
As produced water and watercut increased, water quality was maintained consistently below the required level, Fig. 6. Apart from one unrelated incident, the oil and grease content of the overboard water was maintained below the required limit while continuously treating.
 |
|
Fig. 6. As the amount of produced water from the well and the watercut increased, the water quality was maintained consistently below the required level.
|
|
Dry tree. A field trial was performed in March 2007 to test the oil and water quality of the new chemistry against first-generation AA-LDHI chemistry at identical dosages.
The first-generation chemistry tested up to 1% BS&W during the field trial, while the new chemistry performed around 0.2% BS&W. First-generation chemistry had a TOG content above 500 ppm during the field trial, while the new chemistry had TOG values around 100 ppm.
Since the conversion to the new chemistry, the material’s ability to prevent hydrate formation has been verified with numerous shut-ins and re-starts without impacting oil and water quality. One shut-in lasted five days, and the well was protected by the new chemistry.
CONCLUSIONS
Two field studies show significant oil and water quality improvements over first-generation AA-LDHI chemistry at high watercuts, up to 80%. The new AA-LDHI is predominantly oil soluble, which allows for improved environmental/toxicity performance and oil/water quality. It also has improved performance for subcooling conditions.
The new chemistry has proven performance to prevent hydrates in a continuous wet-tree application and in numerous shut-in/restarts for both a dry-tree and wet-tree applications even at high watercuts without impacting oil/water quality. The effective dosage range of 0.5-2% by volume based on water production is significantly lower than conventional chemical methods.
Improved water quality reduces the consumption and cost of water- and oil-polishing chemicals. The new AA-LDHI chemistry has proven performance for use in deepwater applications with pumping over long distances through umbilicals and/or small-diameter tubing. It meets all requirements for temperature stability, cleanliness, high-pressure viscosity and materials compatibility.
With this new chemistry, companies will be able to produce wells with high watercuts, where oil would have been lost or considered unrecoverable due to economics or technical limitations, adding significant value. This technology can make older wells economical again and allow producers to recover more oil. 
ACKNOWLEDGEMENT
The authors thank Panchalingam Vaithalingam, Scot Bodnar, Johann Venter, Ann Davis, Brad Smith, Mike Rudel, Tom Williams, Alvin Webb, Sharon Simkins, Shawn Gao, Barry Broussard, Tres Wegmann, Kenny VanNess, Tom Kowalski, Glenda Vale, Mike Ive and others for their contributions, without which this new chemistry development would not have been possible. This article was derived from OTC 19505, which was presented at the 2008 Offshore Technology Conference held in Houston, May 5-8, 2008.
LITERATURE CITED
1 Kelland, M. A., “History of the development of low dosage hydrate inhibitors,” Energy and Fuels, 20, No. 3, 2006, pp. 825-847.
2 Mehta, A. P., Hegert, P. B., Cadena, E. R. and J. P. Weatherman, “Fulfilling the promise of low dosage hydrate inhibitors: Journey from academic curiosity to successful field implementation,” OTC 14057 presented at the Offshore Technology Conference, 2002.
3 Cowie, L., Bollawaram, P., Erdogmus, M., Johnson, T. and W. Shero, “Optimal hydrate management and new challenges in GOM deepwater using ‘best in class’ technologies,” OTC 17328 presented at the Offshore Technology Conference, 2005.
4 Zabaras, G. J. and A. P. Mehta, “Effectiveness of bullheading operations for hydrate management in DVA and subsea wells,” OTC 16689 presented at the Offshore Technology Conference, 2004.
|
THE AUTHORS
|
|
|
Rama Alapati earned an MS degree in chemical engineering from the University of Louisiana. He has worked in the oil and gas industry for 15 years and holds two US patents. His expertise includes the areas of flow assurance, reservoir fluid studies, design of innovative oilfield equipment, and chemical injection system design. Alapati is the Engineering Manager for Champion Technologies’ Technical Support and Development Team.
|
|
|
|
Jeremy Lee earned a bachelor’s degree in chemical engineering from Louisiana Tech University. He has extensive experience in oil-water separation, flow assurance, asset integrity and other production concerns, and supports major Capex projects through design, construction and startup phases. Lee is a Deepwater Applications Engineer with Champion Technologies.
|
|
|
|
David Beard earned a BS degree in industrial technology engineering from the University of Southern Mississippi. He has worked in the oil and gas industry for 30 years, which includes work on major projects: BP’s Holstein, Atlantis and Mad Dog; ExxonMobil’s Kizomba A and B and Diana Hoover; Chevron/Texaco’s Petronius; and Placid Oil’s Black Lake Field in North Louisiana. Beard is an Area Manager responsible for Champion Technologies’ Deepwater Group assigned to BP.
|
|
|












