SPECIAL FOCUS: PRODUCTION TECHNOLOGY
Making microbial methane work: The potential for new biogenic gas
In situ, biogenic methane generation is a potentially immense resource that so far has eluded commercial development. Recent research suggests what some of the limiting factors may be, and how to use them to maximize the resource’s potential.
Patrick C. Gilcrease, South Dakota School of Mines and Technology; and George W. Shurr, GeoShurr Resources, LLC.
Recent studies have shown that viable methanogenic microorganisms are still present in a number of unconventional biogenic gas reservoirs. This suggests the potential for the production of new methane from existing coal, shale and oil deposits in real time, resulting in a renewable source of natural gas.
Various non-organic nutrients may need to be introduced into deposits to stimulate in situ methane production. Such nutrients may not be the only limiting factor, as laboratory studies indicate that the surface area and bioavailability of the organic substrates can also influence methane production rates. In this case, conventional reservoir treatments such as fracing, steam flooding, acid treatments and the use of surfactants may be beneficial.
A basic knowledge of the environmental conditions needed for microbial methane production may also be used to screen for sites with the best potential for real-time in situ methane production. The decision to use indigenous or foreign microorganisms and the potential impact of current dewatering practices must also be considered.
MICROBIAL METHANE IN NATURE
Biogenic methane is produced when specific microorganisms metabolize organic compounds under strict anaerobic conditions. Its presence in the natural environment was first documented in 1776 by Alessandro Volta, who collected “combustible air” from a shallow lake by stirring the bottom with a cane.1 The same process can potentially occur in any organic-rich, anaerobic environment, such as rice paddies or swamps, or within landfills. Huge quantities of biogenic methane are also produced by microbes in the rumen of cattle and the hindgut of termites. Total global emissions of biogenic methane from these various sources have been estimated between 16 and 35 Tcfy.2
The same microbial process takes place in modern wastewater treatment systems during anaerobic sludge digestion, converting a portion of the solid waste stream into a valuable methane byproduct. The production of microbial methane from landfills and anaerobic sludge digesters has been studied in some detail, as there is economic benefit from optimization of methanogenesis.
Microbiology of methanogenesis. Methanogens are a special class of microorganisms that produce methane gas as the end product of their metabolism. They are the strictest of anaerobes, as they will not grow or produce methane in the presence of oxygen; the redox potential of their environment needs to be less than -300 mV.3 These organisms use a limited number of simple carbon compounds as substrates, most commonly H2-CO2 and acetate. Other methanogenic substrates include formate, methanol, methylamines, dimethyl sulfide, ethanol and isopropanol;3,4 thus, for the conversion of complex organic substrates (such as coal) to methane, other microbes known as fermentative and acetogenic bacteria must also be present.
This collection of different microbial species in which each organism benefits from the others is referred to as a consortium. First, hydrolytic bacteria break down complex organic substrates; hydrolysis products are then used by fermentative bacteria to produce acetate, longer-chain fatty acids, carbon dioxide and hydrogen, Fig. 1. Homoacetogenic bacteria can consume hydrogen and carbon dioxide to produce more acetate. For ligneous substrates like coal, hydrogen-utilizing acetogens can also convert hydroxylated aromatic compounds to acetate. Hydrogen-producing acetogens (syntrophs) convert fatty acids, alcohols and some aromatic and amino acids to the hydrogen, carbon dioxide and acetate needed by the methanogens.5
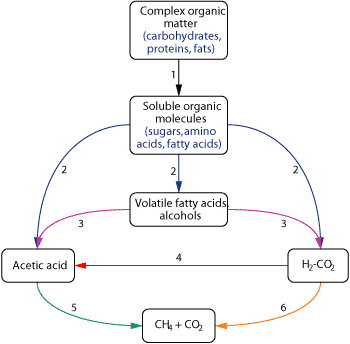 |
Fig. 1. Pathways for microbial methane production from organic matter. Modified from Winfrey and Madigan et al.32,33 Numbered reactions are catalyzed by the following members of the microbial consortium: 1) cellulolytic and other hydrolytic bacteria; 2) fermentative bacteria; 3) syntrophic, hydrogen-producing acetogens; 4) hydrogen-utilizing homoacetogens; 5) acetoclastic methanogens; and 6) CO2-reducing methanogens.
|
|
Microbial methane during coalification. As the process of coalification involves the burial of plant material to produce an anaerobic, organic-rich environment, it is not surprising that biogenic methane production occurs during coal formation. Methane generated from peat during early coalification is referred to as primary biogenic methane. As burial rates and bed pressures are generally low during this step, it is believed that most primary biogenic methane is lost to the atmosphere. Microbial methane can also be generated during deposition of marine sediments.6 This has been documented in deep sea environments as well as shallow marine settings.7,8 In this case, primary methane can be trapped in the rocks, contributing to producible natural gas reserves.9 When higher-rank coals are uplifted and exposed, meteoric recharge can transport microbes into permeable coalbeds, resulting in the production of secondary biogenic methane.10,11
Elemental analyses of methane for carbon-13 and deuterium isotopes can be used to interpret the origin of produced gas, where compositions are expressed as ratios relative to analytic standards. Observed compositions tend to fall into two separate clusters on a crossplot of the carbon isotope ratio and the deuterium isotope ratio. One cluster is characteristic of the acetate pathway for microbial methane, and the other of the CO2 reduction pathway.12 Mixed gases may display intermediate ranges of the ratios, but the two relatively distinct end-member components appear to have limited ranges.
Isotopic analysis of produced methane has shown various coalbed methane plays to be biogenic rather than thermogenic in origin.10,11 Important economic accumulations of microbial methane are found in both fractured marine shales and coalbeds, some of which are dominantly late-generation or secondary microbial methane.13-16 “Sweet spots” within CBM plays are commonly areas of secondary enrichment by late-stage methanogenesis.11 Indeed, secondary biogenic gas has been observed to form in coal desorption canisters over a period of two years.17 This anecdotal evidence for real-time generation is augmented by more stringent laboratory investigations.
The production of secondary biogenic methane from higher-rank coals (lignite and above) implies that certain coal components can be used by microbial consortia, ultimately to produce methane. A number of laboratory studies have demonstrated the microbial conversion of coal to methane when key microbial nutrients are combined with coal and known methanogenic cultures under strict anaerobic conditions.18,19 Cultures obtained from termite guts, sewage treatment plants and animal wastes have all tested positive for methane production from coal. It should be noted that the bioavailability of coals tends to decrease as rank increases.
In other studies, produced water or drilled cores were used to supply the microbial consortia for these experiments; in this case, positive results indicate the in situ presence of living microbes in real time.23,25,28 Viable consortia have been detected in water/coal samples from the Powder River and Michigan Basins, supporting isotopic evidence that the methane in these reservoirs is biogenic. Laboratory methanogenesis rates from indigenous consortia are more likely than those from foreign consortia to be representative of potential in situ activity.
STIMULATION OF REAL-TIME COAL METHANOGENESIS
The presence of biogenic methane, combined with the detection of viable microbial consortia known to produce methane from coal, suggests that methane production may be occurring in real time, and that this process could be stimulated or enhanced in situ. This concept was first proposed by Andrew R. Scott, who proposed the acronym MECoM (Microbially Enhanced Coalbed Methane) to describe processes in which bacterial consortia and/or nutrients are introduced to enhance coalbed methane recovery.20 Such processes may generate new methane and may also improve coalbed permeability via the consumption of coal and pore-plugging waxes.
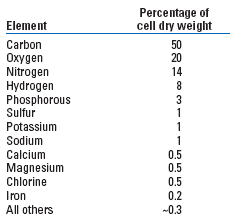
Microbial nutrients. To determine what chemicals are needed for MECoM treatments requires an understanding of the microbes’ basic nutrient requirements. While the microbial world exhibits amazing diversity in its ability to metabolize different substances, there are basic nutrient requirements that are common to any living cell. The old adage “You are what you eat” is true for microbes as well as humans; as such, we can begin to appreciate the nutritional requirements of microbes by examining the elemental analysis of a typical microbial cell, Table 1.
| TABLE 1. The elemental composition of E. coli31 |
 |
|
As coals commonly contain only 1-2% nitrogen, microbes using coal as the primary substrate are likely to be nitrogen limited. Protein digests, ammonium salts and urea are all used as supplemental nitrogen sources in large industrial fermentations. Nitrate is not recommended as a nitrogen source for methanogenic cultures, as it can stimulate the growth of organisms that will out-compete the methane producers for available nutrients. The oxygen content of coals varies from 17 to 27% for lignite coals, and is less for higher-rank coals. Microbial activity associated with coals has been observed to decrease with decreasing oxygen content of the coals.20 The microbes may become limited for the elemental oxygen needed to construct new cell material, and/or this may reflect the poor biosolubility of low-oxygen coals.
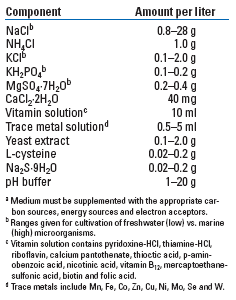
A generic recipe for cultivating microorganisms (Table 2) is used to supplement a primary carbon source (glucose is commonly used in laboratory/industrial fermentations), which provides the C, O and H macronutrients shown in Table 1. Methanogens generally require as a primary carbon and energy source H2-CO2 or acetate, which are produced by other members of the consortium that can use complex organic substrates such as coal, Fig. 1. Acetate not only is used for the production of methane and cellular energy, but also serves as an important building block for new cell biomass in many methanogens.3,21 A mineral solution (first six components in Table 2) provides most of the other macro- and micro-nutrients.
| TABLE 2. Basal microbiological mediuma,21 |
 |
|
The three-dimensional structure of enzymes is critical for catalytic activity; often a second, smaller molecule, known as a cofactor, must combine with the enzyme to achieve an active form. Vitamins and trace metals are often needed in small concentrations as cofactors; for example, methanogens growing on H2-CO2 use metallo-enzymes containing iron, cobalt, nickel and molybdenum and/or tungsten.22 While metals may be essential at low concentrations, many metals (Ni, Co, Cu, Zn, etc.) will become toxic at higher concentrations.
Yeast extract is often added to industrial fermentations in small amounts (as low as 50 mg/L) as a “magic fairy dust” when key growth factors are missing or unknown. It provides a complex mixture of acetate, amino acids, nitrogen bases, vitamins and trace metals needed for almost any microbial strain. Finally, cysteine and sodium sulfide are added as reducing agents to ensure that the redox potential is sufficiently low for methanogens. Strict anaerobic conditions must be maintained throughout the culturing process, Fig. 2.
 |
Fig. 2. A gassing manifold is used to “feed” methanogenic cultures by adding an H2-CO2 headspace under pressure. Aseptic, anaerobic techniques must be strictly adhered to when culturing methanogenic consortia in the laboratory.
|
|
Temperature, pH and salinity. Environmental parameters such as temperature, pH and salinity all have an effect on microbial metabolism and growth. In general, cellular structures are held together by relatively weak chemical bonding forces that are easily disrupted by environmental changes. Individual species tend to have relatively narrow ranges in which they can survive and grow. However, living microorganisms have been discovered in the most extreme natural environments, as each environment selects for those microbes that are best suited to it. Microbial activity increases exponentially with temperature to a point, but if the temperature is too high, cells die when enzymes are denatured and/or cell membranes are disrupted. Methanogens have been found in natural environments ranging from 2°C to over 100°C, but optimum temperatures for methanogenesis are often near 35°C.3
A methanogenic consortium was enriched from water produced in the Powder River Basin at 17.5°C; when grown on Wyodak coal in the laboratory, methane production rates were three times greater at 38°C than at 22°C.23 While cell metabolism and growth were likely enhanced at the warmer temperature, the aqueous solubility of coal will also increase with temperature, making it more bioavailable. Coalbed reservoirs at higher temperatures may have a greater potential for enhanced microbial methane production, as the environment may have selected for microbes with higher temperature optima and, thus, higher metabolism and growth rates.
Microbes typically require an intracellular pH close to neutrality, but cannot maintain this when the environmental pH becomes too high or too low. Most methanogens have optimal activity when the environmental pH is near 7.0, but microbial methane production has been observed at pH levels as low as 3.0 and as high as 9.2.3 For the Powder River Basin consortium discussed above, methane production rates from Wyodak coal were highest when the culture medium pH was 6.1 as compared with pH of 7.6 and 8.3. The lower pH level may have enhanced the intrinsic activity of the microbes and/or the aqueous solubility of the coal; acids are known to disrupt ionic bridges and hydrolyze ester or ether bonds within the coal matrix.24
Environmental salinity affects the ability of microbes to maintain the right amount of intracellular water. High-salinity environments will cause the cell to lose water and dry up; this is why salt is so effective for the preservation of meat and other foods. Conversely, cells will fill up with water and burst if external salinity levels are too low. Methanogens are found in salinity environments ranging from freshwater to hypersaline, where the sodium ion concentration ranges from 1 mM to 3M, respectively.3 A recent study investigated the effect of salinity on methane production from Antrim Shale enrichment cultures, where microbial consortia were collected from well water along a salinity gradient ranging from 47 mM to 1.8 M Na+.25 While methane production was observed from all samples except one with the highest salinity, methane production was maximized when the enrichment medium salinity closely matched the salinity of the original water sample.
As salinities increase, so does the cellular energy “cost” of maintaining an intracellular osmotic pressure greater than that of the environment. As such, methanogens that use acetate (energy yield of -31 kJ/mol) are limited to lower-salinity environments, while high salinity favors methanogens that can use methanol, methylated amines and/or methylated sulfides (energy yields of -79 to -191 kJ/mol). The presence of methylated amines and methylated sulfides is expected in saline/hypersaline environments, as they are the breakdown products of cellular osmoprotectants.3 In addition, these methylated substrates cannot be used by sulfate-reducing bacteria, which would otherwise out-compete methanogens in marine environments where sulfate is present.
Surface area limitations. Assuming that microbial metabolism and cellular growth can be stimulated through the addition of key nutrients to the reservoir, real-time in situ methanogenesis of coal or other organic source rocks may ultimately be limited by surface area. The diameter of many coal pores (which range from 0.04 to 30 µm) is too small for microbial cells (diameter 0.2 to 6.0 µm); thus, microbial access is limited to the cleat surfaces of the coal.20,24 The microbial uptake of coal nutrients involves dissolution at the coal-water interface followed by diffusion through the aqueous phase to the degrading microbes. Nutrient concentrations will be highest at the coal surface, and microbes nearest the surface will be capable of faster uptake. For this reason, the rate and quantity of methane generated from coal may depend on the surface area of coal that is available to the microorganisms, and the insolubility and impermeability of coal may represent major constraints.
The effect of coal surface area on methanogenesis rates is demonstrated by Powder River Basin enrichment cultures that were provided Wyodak coal of different particle sizes as the sole carbon-energy source, Fig. 3. The maximum methane production rate for the small-particle cultures (80-140 mesh) was 5 cu ft per ton of coal per day, which was 214% greater than the maximum rate for the large (12-20 mesh) particles.23 A plot of methane production rate vs. external coal particle surface area exhibited a linear, positive trend, which is expected when the microbial process is limited by the dissolution of coal substrates. If this holds true for in situ bioconversion processes, then coal surface area can be used to extrapolate laboratory rates to subsurface rates.20
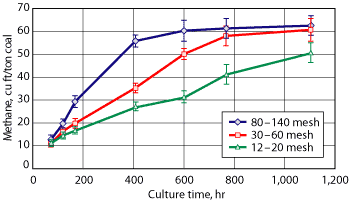 |
Fig. 3. Biomethane production from Wyodak coal as a function of coal particle size. From Green et al.23
|
|
Solvents and surfactants. For methanogenesis processes that are limited by coal dissolution rates, solvents and surfactants may provide a means to enhance these rates. Solvents and surfactants often represent a double-edged sword when used in biological systems with solid/sparingly soluble substrates. On one hand, these agents can enhance the aqueous solubility of coal compounds, which increases the rate of dissolution into the aqueous medium. These compounds may also enhance the uptake of hydrophobic substrates like coal through the microbial cell wall or membrane, further enabling metabolism. On the other hand, these same compounds can be toxic to microorganisms, especially at higher concentrations, as they may denature/dissolve critical cell structures. Surfactant micelles have been shown to reduce bioavailability in some biodegradation systems;26 furthermore, organic solvents injected into coalbed methane wells to remove coal have lowered permeability and damaged the reservoir.20
Laboratory experiments have shown that certain solvents and surfactants added at the right concentration can significantly enhance coal bioconversion to methane; unfortunately, the value of the additional methane produced was less than the cost of these expensive agents. The effect of solvents and surfactants tends to be highly substrate specific and microbial species specific, but further research may discover agents that are cost-effective MECoM treatments. A number of microorganisms are known to produce their own biosurfactants;27 if surfactant-producing organisms could be introduced to the coalbed reservoir, this might prove to be a less expensive alternative.
USE OF CONVENTIONAL RESERVOIR TREATMENTS
For the in situ stimulation of microbial methane generation, the addition of non-carbon nutrients such as those given in Table 2 will be important, and will most likely be site specific, depending on the type of coal, reservoir water chemistry and species of microbes present. Field results for in situ stimulation are just beginning to come in, as Luca Technologies recently completed a field trial in the Powder River Basin. While the details of the trial are not yet available, the company reports that it “has yielded encouraging results.”34 However, if nutrient addition is successful in stimulating microbial growth and metabolism, at some point the system will become surface-area (i.e., dissolution rate) limited.
At this point, a number of conventional oil and gas reservoir treatments could potentially enhance in situ methanogenesis. Fracing operations could certainly be used to enhance coal/source rock surface areas needed for microbial attack, but could this be done without degrading the reservoir transport properties? While it may not be economical to warm a substantial volume of the reservoir with steam flooding to enhance microbial metabolism and growth rates, could steam could be used to help dissolve key coal components to be used by viable microbes downstream of the heated zone? Similarly, it may not be economical to alter the pH of the entire reservoir via the pumping of acids or bases, but could coal components dissolved in a low- or high-pH zone be transported to neutral-pH zones where microbial methane activity is high? There are numerous research and engineering challenges associated with these questions, but the prospect of using conventional reservoir treatments for the stimulation of unconventional gas is quite exciting.
That said, one “conventional” practice for unconventional gas production could potentially hinder or even prevent future microbial stimulation efforts. The dewatering of coalbed methane reservoirs is used to lower reservoir pressures to the point that methane desorbs from the coal or other source rock, allowing gas production to occur. As microbes are inherently aquatic organisms, water is essential to their survival and growth. Does the removal of water from the reservoir adversely affect the indigenous microbial population, or does enough residual water remain in the cleats/pores to sustain these organisms? Will dewatering change the reservoir properties in a manner that will hinder the transport of microbial nutrients in future stimulation treatments? If a “refilling” of the reservoir is necessary, can this be done in a manner that does not introduce oxygen or other compounds that are toxic to methanogens? These represent critical questions that also need to be addressed in future R&D efforts.
FOREIGN VS. INDIGENOUS MICROBES
While the definition of MECoM given above includes the possibility of adding foreign microbes to a coal/shale/organic-bearing reservoir, this may or may not be necessary to stimulate microbial methanogenesis. Limited sampling studies of the Powder River and Michigan Basins have shown that viable methanogenic populations are already present.23,25,28
In situ biodegradation studies frequently show that contaminated sites will select for organisms that can degrade the contaminant, and these indigenous populations will perform as well as or better than any foreign microbes that are introduced to the site.29,30 However, in one laboratory study, a methanogenic consortium derived from a wood-eating termite was used to convert a Texas lignite coal to methane;18 after correcting for methane produced from non-coal nutrients, maximum methane production rates of 379-531 cu ft per ton of coal per day were observed for coal particle sizes ranging from 28 to 325 mesh. These rates were significantly higher than any of the rates observed in the laboratory using indigenous consortia from the Powder River Basin. While the solubility and bioavailability of the lignite coal used in the study is probably greater than the sub-bituminous coal used in Powder River study, the results still raise the possibility that this termite-derived consortium is more robust than the native consortium. Whether this foreign culture could outcompete native methanogens in situ remains to be seen. Like all of the potential stimulation treatments discussed here, the optimal scenario is likely to be site specific.
CONCLUSIONS
Research studies to date suggest the potential to generate new microbial methane from coal or other organic source material in real time via the addition of microbial consortia and/or key microbial nutrients. Such MECoM processes could generate significant new methane reserves; a 3% conversion of known North American hydrocarbon deposits would produce 1,000 Tcf of natural gas.28 While knowledge of microbial consortia from other methanogenic environments (termites, sludge digesters, etc.) provides a basis for understanding the MECoM process, significant R&D challenges remain.
The varied geology, chemistry and microbiology of different production sites will most likely demand site-specific strategies. If microbial activity can be stimulated in situ, at some point the methane generation process will likely be limited by the surface area and aqueous solubility of the coal/source rock. At this point, conventional stimulation treatments such as steam flooding and fracing may prove beneficial. 
LITERATURE CITED
1 Wolfe, R. S., “An historical overview of methanogenesis,” in Ferry, J. G., ed., Methanogenesis: Ecology, Physiology, Biochemistry and Genetics, Chapman and Hall: New York, 1993, pp. 1-32.
2 Tyler, S. C., “The global methane budget,” in Rogers, E. J. and W. B. Whitman, eds., Microbial Production and Consumption of Greenhouse Gases: Methane, Nitrogen Oxides, and Halomethanes, American Society for Microbiology, Washington D.C., 1991, pp. 7-58.
3 Zinder, S. H., “Physiological ecology of methanogens,” in Ferry, J. G., ed., Methanogenesis: Ecology, Physiology, Biochemistry and Genetics, 1993, pp. 128-206.
4 Widdel, F., “Growth of methanogenic bacteria in pure culture with 2-propanol and other alcohols as hydrogen donors,” Applied and Environmental Microbiology, 51, No. 5, 1986, pp. 1056-1062.
5 McInerney, M. J. and M. P. Bryant, “Review of methane fermentation fundamentals,” in Wise, D. L. ed., Fuel Gas Production From Biomass, CRC Press, Boca Raton, Fla., 1981.
6 Parkes, R. J. et al., “Biogeochemistry and biodiversity of methane cycling in subsurface marine sediments,” Environmental Microbiology, 9, No. 5, 2007, pp. 1146-1161.
7 Sivan, O., Schrag, D. P. and R. W. Murray, “Rates of methanogenesis and methanotrophy in deep-sea sediments,” Geobiology, 5, No. 2, 2007, pp. 141-151.
8 Lin, C. M. et al., “Geology and formation mechanism of late Quaternary shallow biogenic gas reservoirs in the Hangzhou Bay area, eastern China,” AAPG Bulletin, 88, No. 5, 2004, pp. 613-625.
9 Shurr, G. W. and J. L. Ridgley, “Unconventional shallow biogenic gas systems,” AAPG Bulletin, 86, No. 11, 2002, pp. 1939-1969.
10 Scott, A. R., Kaiser, W. R. and W. B. Ayers, Jr., “Thermogenic and secondary biogenic gases, San Juan basin, Colorado and New Mexico - implications for coalbed gas producibility,” AAPG Bulletin, 78, No. 8, 1994, pp. 1186-1209.
11 Faiz, M. and P. Hendry, “Significance of microbial activity in Australian coalbed methane reservoirs - a review” Bulletin of Canadian Petroleum Geology, 54, No. 3, 2006, pp. 261-272.
12 Whiticar, M. J., Faber, E. and M. Schoell, “Biogenic methane formation in marine and freshwater environments: CO2 reduction vs. acetate fermentation-isotopic evidence,” Geochimica et Cosmochimica Acta, 50, 1986, pp. 693-709.
13 Martini, A. M. et al., “Microbial production and modification of gases in sedimentary basins: A geochemical case study from a Devonian shale gas play, Michigan basin,” AAPG Bulletin, 87, No. 7, 2003, pp. 1335-1375.
14 Ayers, W. B., “Coalbed gas systems, resources, and production and a review of contrasting cases from the San Juan and Powder River basins,” AAPG Bulletin, 86, No. 11, 2002, pp. 1853-1890.
15 Stricker, G. D. et al., “Shallowest coalbed methane accumulation in the west: It’s all about water and microbes in the Powder River basin,” AAPG Abstracts, 2006, pp. 102.
16 Gorody, A. W., “The origin of natural gas in the tertiary coal seams on the eastern margin of the Powder River Basin,” in Miller, W. R., ed., Coalbed Methane and Tertiary Geology of the Powder River Basin: Wyoming Geological Association Guidebook, 50th Annual Field Conference, 1999, pp. 89-101.
17 Barker, C. E. and T. Dallegge, “Secondary gas emissions during coal desorption, Marathon Grassim Oskolkoff-1 well, Cook Inlet Basin, Alaska: Implications for resource assessment,” Bulletin of Canadian Petroleum Geology, 54, No. 3, 2006, pp. 273-291.
18 Harding, R., et al., “Biogasification of low-rank coal,” Arctech, Inc. for Electric Power Research Institute, Chantilly, Va, 1993.
19 Volkwein, J. C., et al., “Biological production of methane from bituminous coal,” Fuel Processing Technology, 40, 1994, pp. 339-345.
20 Scott, A. R., “Improving coal gas recovery with microbially enhanced coalbed methane,” in Mastalerz, M., Glikson, M. and S. D. Golding, eds., Coalbed Methane: Scientific, Environmental and Economic Evaluation, Kluwer, Dordrecht, The Netherlands, 1999.
21 Tanner, R. S., “Cultivation of bacteria and fungi,” in Hurst, C. J. et al., eds., Manual of Environmental Microbiology, ASM Press, Washington D.C., 2002, pp. 62-70.
22 Thauer, R. K., Hedderich, R. and R. Fischer, “Reactions and enzymes involved in methanogenesis from CO2 and H2,” in Ferry, J. G., ed., Methanogenesis: Ecology, Physiology, Biochemistry and Genetics, 1993, pp. 209-252.
23 Green, M. S., Flanegan, K. C. and P. C. Gilcrease, “Characterization of a methanogenic consortium enriched from a coalbed methane well in the Powder River basin,” International Journal of Coal Geology, 2007, in review.24 Faison, B. D., “The chemistry of low rank coal and its relationship to the biochemical mechanisms of coal biotransformation,” in Crawford, D. L., ed., Microbial Transformations of Low Rank Coals, CRC Press, Boca Raton, Fla., 1992.
25 Waldron, P. J. et al., “Salinity constraints on subsurface archaeal diversity and methanogenesis in sedimentary rock rich in organic matter,” Applied and Environmental Microbiology, 73, No. 13, 2007, pp. 4171-4179.
26 Mihelcic, J. R. et al., “Bioavailability of sorbed- and separate-phase chemicals,” Biodegradation, 4, 1993, pp. 141-153.
27 Youssef, N. et al., “In situ biosurfactant production by Bacillus strains injected into a limestone petroleum reservoir,” Applied and Environmental Microbiology, 73, No. 4, 2007, pp. 1239-1247.
28 Finkelstein, M. et al., “Buried hydrocarbons: A resource for biogenic methane generation,” World Oil, August 2005, pp. 61-67.
29 Baker, K. H., “Bioremediation of surface and subsurface soils,” in Baker, K. H. and D. S. Herson, eds., Bioremediation, McGraw-Hill, N.Y., 1994.
30 Cookson, J. T., Jr., Bioremediation Engineering Design and Application, McGraw-Hill, N.Y., 1995.
31 Luria, S. E., “The elemental composition of E. coli,” in Gunsalus, I. C. and R. Y. Stanier, eds., The Bacteria, Academic Press, N.Y., 1960.
32 Winfrey, M. R., “Microbial production of methane,” in Atlas, R. M., ed., Petroleum Microbiology, Macmillan, New York, 1984, pp. 153-220.
33 Madigan, M. T., Martinko, J. M. and J. Parker, Brock’s Biology of Microorganisms, 10th Ed., Prentice Hall, Upper Saddle River, N.J., 2003.
34 Finkelstein, M., vice president-Biosciences for Luca Technologies, interview by author, Oct. 9, 2007.
|
THE AUTHORS
|
|
Patrick C. Gilcrease is an assistant professor of chemical engineering at the South Dakota School of Mines and Technology. He earned a BS degree in chemical and petroleum-refining engineering from the Colorado School of Mines in 1987. After working in the chemical industry for four years, he obtained his MS and PhD degrees in chemical engineering from Colorado State University in 1993 and 1997, respectively. During his 16 years in academia, he has researched the bioremediation/biocatalysis of solid/sparingly soluble substrates such as coal and lignocellulose.
|
|
|
George W. Shurr was a geology professor for 30 years while working part time with the South Dakota Geological Survey, the US Geological Survey, and consulting. In 1998 he started GeoShurr Resources, LLC, which focuses on exploration and development of shallow gas. Visit www.geoshurr.com for current activities and abstracts.
|
|
|








