Buried hydrocarbons: A resource for biogenic methane generation
Unconventional ResourcesBuried hydrocarbons: A resource for biogenic methane generationJust 3% of Mother Nature’s vast buried coal, carbonaceous shale and residual oil could be treated by biogenesis to produce 1,000 Tcf of methane.Buried hydrocarbons, especially those that cannot be easily and economically recovered, represent a universal opportunity to meet future natural gas needs. We have come to accept landfill gas microbially generated from municipal wastes as a viable means of methane generation. However, we fail to recognize massive coal, shale and residual oil deposits as vast supplies of old biomass that nature has anomalously concentrated for us, just as we concentrate municipal waste in landfills. These residual oils, coal and carbonaceous shales are abundant in nature, and the results outlined below demonstrate that these resources can be readily transformed to methane in the lab. Because of the prodigious amount of substrate available for conversion to methane, only a small portion of this resource needs to actually be converted in the field to have a large economic impact. The indigenous microbial communities responsible for this conversion perform the necessary metabolic reactions in an environmentally benign fashion in real time. Luca Technologies (Luca) has obtained and characterized samples from over 25 geologically distinct locations in North America. Many of these are – or have the potential to become – Geobioreactors.* The company is now finalizing plans to begin in situ field trials to link favorable lab findings with field implementation. This effort can be carried out using the incremental economics provided by coalbed methane (CBM), shale gas, and oil fields that have already been drilled, based on primary recovery economics. Extending the lives of such wells gives us more use from existing infrastructure, while limiting further surface impacts. INTRODUCTION As unconventional natural gas production becomes an ever-increasing component of natural gas supply, and therefore more “conventional,” we must look toward the application of new technologies and innovations to these unconventional resources to help us keep pace with dwindling supplies and rising demand. While constituting less than 10% of the US’s overall natural gas production, biogenic methane is a fast – growing segment of our natural gas supply. The increasing number of biogenic gas plays such as the Powder River basin (PRB) of Wyoming, the Antrim Shale in Michigan, and the emerging CBM projects in Alberta, suggest that we are at the beginning of a large growth curve. An understanding of how this methane is generated and the parameters that affect its rate of generation has been the focus of an intense research effort by a number of institutions. Lab studies and field results indicate that a large untapped resource base exists that could substantially increase and extend the world’s natural gas supplies. Only 25-40% of the oil in a typical reservoir can be cost-effectively brought to the surface using current waterflood and enhanced oil recovery technologies. Similarly, most coal cannot be mined economically due to depth constraints; and the mining of organic-rich shales to produce oil has so far proven to be uneconomic. This trapped oil, buried coal and carbonaceous shale represent a vast energy resource that is largely unrecoverable with existing technologies. The microbial creation (“biogenesis”) of methane was first recorded over 225 years ago.1 It has since come to be recognized as a common, natural process in the biosphere and geosphere. Due to the presence of huge resources of hydrogen and carbon in buried organic materials, the potential exists for the generation of immense volumes of methane if biogenesis can be “domesticated.” Microbial conversion of residual oil, coal and kerogen to methane (natural gas), in real time, has the potential to create a substantial source of natural gas. Presented here is experimental evidence that indigenous microbial communities in some oil reservoirs and coal seams can be stimulated to produce economically significant volumes of methane. THE SIGNIFICANCE OF BIOGENESIS A previous USGS study estimated that biogenic methane comprised 22% of the methane known to the petroleum industry.2 When the methane estimated to exist in gas hydrates is added, greater than 50% of world methane resources is thought to be biogenic in origin.3 While estimates of the total hydrocarbon reserves in the US vary widely, a conservative estimate of the substrate in place (SIP) for possible conversion to methane is shown in Table 1. This table uses the ultimate analysis, or chemical composition, of each of these substrates to determine the amount of sequestered hydrogen they contain and, therefore, the absolute upper limit of methane which could be produced from them microbially. The kerogen, or carbonaceous portion of the shales, corresponds to about 2,000 billion short tons (BSTs), whereas over 3,000 BSTs of coal exist in the lower 48 states, and about twice that amount is thought to exist in Alaska. Residual oil in the US that does not lend itself to recovery with known technologies is estimated to total about 262 Bbbl. While all of this substrate cannot possibly be converted to methane, the amount of hydrocarbon resource that can be managed for the production of microbial methane is enormous. Conversion of just 3% of these hydrocarbon substrates to methane translates to 1,000 Tcf of natural gas, over 40 years’ worth of US natural gas supply, at the present usage rate of 23 Tcf per annum. Equally significant are the rates of methane generation. While the conventionally recognized thermogenic process requires millions of years to bury and transform organic material into oil and gas, biogenesis can be observed as occurring in real time in anaerobic landfills, lab experiments, and occasional coal samples undergoing desorption testing. The rates can be striking. For example, a large landfill in Canada has produced more than 26 MMcfd of low-btu gas.4 Microcosm studies of PRB coal samples in Luca’s lab, using formation coal and water samples without chemical additives, have produced at rates which would equal 66 Mcf/day if they could be replicated over 40 acres in a typical 50-ft-thick PRB coal seam. Hydrogen and carbon resources in this same 50-ft- thick coal over 40 acres would support this rate for almost 250 years, if 25% of the coal’s hydrogen were liberated and incorporated into methane, or about 1,000 years if 100% conversion could be achieved. Even greater rates are obtained if appropriate nutritional and chemical amendments are added. DOCUMENTED US BIOGENIC METHANE OCCURRENCES Biogenic methane has several recognizable characteristics that distinguish it from thermogenic methane:
In the US, biogenesis is recognized in a number of basins:
In addition to the above, study of the in situ microbial degradation of crude oil indicates that greater than 50% of all oil reservoirs experience some biogenic conversion of oil to methane.14 Real-time biogenesis of methane has been observed in the following substrates:
The above data raise the distinct possibility that vast reservoirs of buried hydrocarbons found in the US, and the rest of the world, can serve as Geobioreactors* for the generation of large quantities of methane. A Geobioreactor is a combination of a hydrogen/ carbon bearing geologic formation with naturally occurring or introduced microorganisms capable of making methane in real time. While all hydrogen/ carbon geologic formations are not active Geobioreactors, Luca has been able to demonstrate that samples taken from some inactive formations are capable of being activated in the lab with either nutritional amendments or cross-inoculations with specific microbial consortia. CREATION OF HYDROCARBONS Plants use the power of sunlight to combine carbon dioxide, water and nutrients into complex sugars, carbohydrates, waxes and lignins. Aquatic phytoplankton, while smaller in mass but more productive, grow on similar constituents. The majority of our hydrocarbon resources were initially made from these photosynthetically-derived materials. Most organic carbon is returned to the atmosphere through the carbon cycle as aerobic (oxidative) decomposed organic matter. However, a small portion (usually less than 1%) of this biomass is preserved in sediments where it typically supports the growth of a diverse community of microorganisms. While only a modest share of this biomass is buried below an oxidative level in any given year, over geologic time, this buried biomass aggregates into vast hydrocarbon deposits. The world’s oil, coal and gas reservoirs have been formed from only a fraction of this anaerobically preserved material. LABORATORY BIOGENESIS STUDIES To understand and enhance in situ biogenic activity, it is necessary to re-create formation conditions in the lab. Great care is taken to avoid chemical and microbial contamination during extraction of samples from the ground, in transport and in the lab. For example:
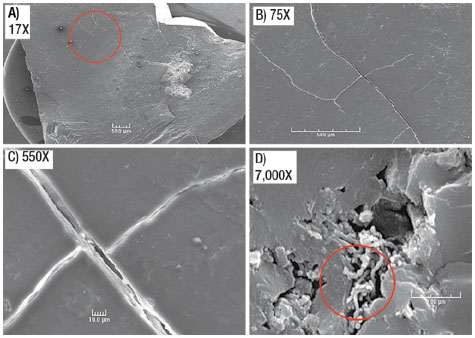
Samples were also prepared for various types of microscopy. In several studies, scanning electron microscopy (SEM) was used to view the three-dimensional structure of a sub-bituminous coal. As the magnification increases to 550X from 17X in panels A-C in Fig. 1, it is apparent that the micro-fracture apertures found within the coal are not only sufficient to allow water and gas to pass through them, but also wide enough for the average microbial cell to move through or congregate within the fracture. This is evident as a colony of microbes is seen at 7,000X in panel D, Fig. 1. Typical fracture apertures range upward from 2 to 10 micrometers and can easily accommodate a wide range of microbes.
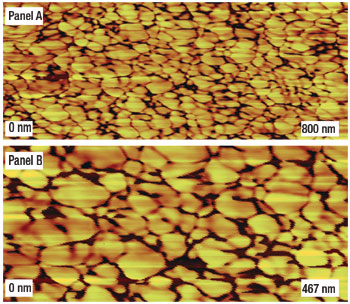
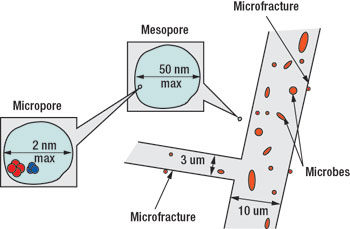
Luca has used atomic force microscopy (AFM) to look at subtle perturbations on the face of sub-bituminous coal surfaces. Rather than a smooth, glassy surface, the high resolution AFM phase image of sub-bituminous coal depicted in Fig. 2 indicates a symmetric, “scale-like” surface, showing the geopolymer molecules which are the building blocks of the coal matrix. The intricate channels between the large geopolymer molecules denote pores through which gas and water molecules (but not microbes) pass. These pore diameters range up to 2 to 50 nanometers (a thousand times smaller than the fractures). A diagram depicting the fractures, meso and micro-pores, methane, water and microbes is shown in Fig. 3. The internal structure of this low-rank coal allows the microbes to travel along and exist in the fractures while water (and nutrients) as well as gases (including methane) can rapidly be transported through pores as well as the fractures.
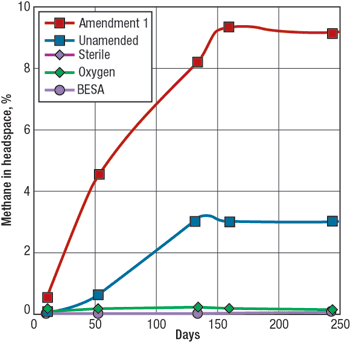
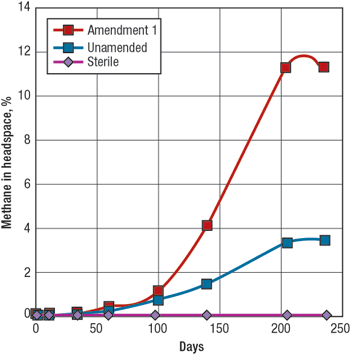
Microbes were also observed growing at the oil-water interface of the Monument Butte experimental oil samples. These microbes were detected using a phase-contrast fluorescent microscope. A number of different dyes were used to differentiate live cells from dead cells. Most of the microbes are rod-shaped with some elongated paired rods evident. While it is likely that a variety of microbes are present, it was not possible to visually identify these particular microbes as to genus and species. Luca’s other types of analyses focus on the identification of key members of the microbial population (consortium), and how the microbes respond to changes in their environment. MICROBIAL METHANE GENERATION In typical experiments, direct measurements of methane concentration are taken over the course of a 3 to 10-month period from aliquots removed from the experimental bottles. The bottles contain substrate (coal or oil), formation water and, in some instances, either stimulatory or inhibitory amendments. In Fig. 4, methane generation is monitored over a 6-month period from samples obtained from the Dietz coals of the PRB. Sample bottles containing coal and formation water (unamended) showed a steady rise of methane in the headspace, peaking at slightly more than 3% at 135 days. When a nutritional enhancer is added (Amendment 1), an immediate and significant boost in methane generation is observed, reading almost 9% methane after 135 days. It should be noted that while the headspace contains only 3% to 9% methane, this methane represents almost 100% of the newly generated gas.
A similar response has been observed in over a dozen coal samples taken from a variety of different coal seams. Control experiments in which the bottle contents are either sterilized, exposed to oxygen, or mixed with a known methanogen inhibitor, bromo-ethanesulfonic acid (BESA),15 all demonstrated little or no methane generation. Oxygen is a known inhibitor of methanogenic bacteria, which are considered among the most oxygen-sensitive of all the anaerobes. Short exposures have been shown to efficiently kill several species.16 Therefore, it is not surprising that sterilization, BESA treatments, or exposure to oxygen will inhibit and perhaps destroy biogenesis. In parallel experiments, a small sample of conventional crude oil recovered from the Bell Creek oil field of the PRB is used in place of coal as substrate for methane generation. Fig. 5 is similar to Fig. 4 in that the sterile control sample generated negligible methane, the unamended sample generates an intermediate level of the methane, and the amended sample generates a substantial amount of methane. Oil from the Monument Butte region of Utah is waxy and contains a high proportion of straight-chain alkanes. This is quite different than the cyclic aliphatic and aromatic molecules typically found in Bell Creek oil.17,18Nevertheless, a very similar methane generation profile was observed in experimental bottles (data not shown). This would indicate that, in addition to areas containing conventional crude oil, areas with large accumulations of waxy oil could prove to be important sites for the bioconversion of residual oil to methane. For example, Daqing field in northeast China is thought to contain 25 Bbbl of waxy residual oil.19,20 This residual oil, left in place after successful waterflood operations, represents a potential biogenic gas substrate of about 80 Tcf at 100% conversion efficiency, compared to total reported Chinese natural gas reserves of between 46 and 53 Tcf.21
Microbial communities. The microbes residing in Geobioreactors make up a community called a consortium. This interdependent community is largely unique for each location, with at least three different members responsible for separate metabolic reactions within each Geobioreactor. These reactions include the initial breakdown of the large hydrocarbon molecule, the hydrolysis of this molecule to simple acids and alcohols, and then the conversion of these smaller chemicals to methane. Any interruption in this chain of events will short-circuit the entire process and prevent methane formation. Interestingly, Luca has observed deficiencies in this microbial progression in samples from some Geobioreactors and has been able to ameliorate the problem with addition of microbes from a different Geobioreactor. RAMIFICATIONS OF EXPERIMENTAL METHANE GENERATION The volumes of methane created during experiments can be extrapolated to scaled-up field dimensions using gas percentage composition measurements, SIPs, and the Ideal Gas Law. For example, the upper section of Table 2 summarizes data from oil wells drilled on a 40-acre spacing to produce from a 20-ft-thick sand with 12% porosity and 40% residual oil saturation, after waterflood, based on data obtained from Monument Butte oil after 87 and 297 days. The calculated Mcf/day/well of methane, 132 and 50 Mcf/d, respectively, is similar to ongoing production rates of these wells.
In the same table, extrapolated rates of methane generation from coal yield values of 66 Mcf/day/well in unamended samples, and 217 Mcf/day/well in amended coal samples over 159 days, over 40 acres. Using these numbers, a 640-acre plot of land containing a 50-ft seam of coal could potentially yield 393 Bcf of methane. This is considerably more than the 2 Bcf expected from a field of this size in a conventional accumulation. The above results are encouraging when extrapolated to in situ field conditions. While the lab conditions have not yet been optimized, field results could surpass or fall short of those obtained in the lab. Nutritional and chemical amendment packages have not been extensively explored, and the same amendments are observed to have different impacts on different substrates. Each Geobioreactor environment is a unique combination of substrate chemistry, water chemistry and microbial community. The ideal set of conditions for stimulating and sustaining methanogenesis will most likely be dissimilar for each substrate, and perhaps vary between different deposits of a similar substrate. Fortunately, the potential volumes of methane available are so large that the expense of defining the most optimal conditions is modest compared to the reward.
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
- What's new in production (February 2024)
- Prices and governmental policies combine to stymie Canadian upstream growth (February 2024)
- U.S. operators reduce activity as crude prices plunge (February 2024)
- U.S. producing gas wells increase despite low prices (February 2024)
- U.S. drilling: More of the same expected (February 2024)
- U.S. oil and natural gas production hits record highs (February 2024)