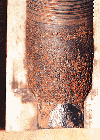drill-cox.html (May-1998)
Drill pipe corrosion: Past, present and futureReview of mud system developments over 40 years, relative to corrosion protection for downhole tubulars, including system descriptions and case historiesTom Cox, M-I L.L.C., Houston
Further discussions include recommendations for handling specimens for analysis, and speculation on the future concerning drill pipe, coatings and techniques. EARLY MUD SYSTEMS In the 1940s and 1950s, some very-high-alkalinity drilling fluids were used. Some were based on the high-pH sodium silicate chemical mixed with a salty base fluid. It seems that drill string damage was quite low due to this elevated pH and the protective nature of the silicate. The drilling fluid systems, however, were not very versatile and this, combined with rig safety concerns, ended the fluid's first-use phase. These fluids brought to the shaker screens "bit-marked" cuttings from the drag bits. The fluids continued to fall out of favor, and not much was heard about them until the late 1990s. They resurfaced in several areas with different additive products now available to allow a more versatile system. Oil-emulsion muds. Red-lime, oil-emulsion fluids were popular in the late 1950s, and appeared to give good drill pipe protection. This was in part due to the high pH (12 to 12.5) and oxygen (O2) scavenging dispersants such as Quebracho. The emulsified diesel formed a protective "oil-wet" film on the metal and allowed the drill pipe to be isolated from the fresh or saltwater environment. This acted as a corrosion inhibitor; and many drill strings were clean, bright and shiny when they were pulled. A low corrosion rate, and minimum pitting was normal with this system.
Brine systems. In areas where "cut-brine" or "field" brine fluids were being used, the associated salt environment and low pH usually showed a high corrosion rate. The brines contained soluble calcium and magnesium; many contained traces of carbon dioxide (CO2) and hydrogen sulfide (H2S) gases. Both of these acid gases, along with the dissolved O2 can contribute to high corrosion rates. In the past, caustic soda, lime and chromates were used for corrosion control in these systems. Sometimes a filming amine inhibitor was slugged down the drill pipe to coat it. "Spray and wipe" attachments were also popular at this time to aid in adding the amines. In many areas today, chromates are considered environmentally sensitive, and their usage has diminished greatly. They were, however, excellent inhibitors; and corrosion rates were high without the chromate ions. Chemical inhibitors containing brine soluble amines were used to lower the corrosion rate of completion and packer fluids as they were needed. Oxygen inhibitor, plastic coating. Internally plastic coated drill pipe reduces O2 attack on the pipe ID and can lengthen string life. Without the protective coating, high-pH and an inhibitor based on organic phosphate esters can be used effectively in the drilling environments to protect the tubulars. In systems containing a high level of soluble cations like calcium and magnesium, the pH cannot be economically raised due to precipitation of insoluble hydroxides. Control of pitting is desired as this will decrease washouts and failure many times. There was some use of the soluble base ammonium hydroxide, especially during sour drill stem tests. But the ammonia release has some environmental safety restraints, and due to new regulations, that product is not used as a chemical of choice anymore. 1960-ERA SYSTEMS During the 1960s, low- or neutral-pH, gypsum-treated fluids were popular as an "inhibited" system to drill with. They were "sulfate-saturated," and contained high filtrate calcium. They sometimes utilized starches for filtration control. During this period, carboxymethyl cellulose (CMC) was popular for filtration control in gyp and seawater fluids. The lignosulfonates were used with desirable improvements in stability. There were many problems with tubular corrosion during this period. Many investigators started to question the lignosulfonate, based on lab testing. The pH values were low, and unsuspecting drillers were plagued with bacterial attack and loss of filtration control, along with corrosion problems. Bacteria can attack the sulfate ion, and also the cellulosics, etc. H2S could be formed with this attack to further aggravate corrosion problems. Biocides were initiated again to reduce the attack. And makeup water was treated to minimize these detrimental bacterial effects. A modern, effective biocide treatment is the addition of 25% glutaraldehyde. Oil muds. These systems were advertised as the muds of choice, as they showed no corrosion, compared to water-based fluids. The protective oil film that formed on the pipe lowered the corrosion rate and the systems were generally considered to be protective. These fluids contained organic chemicals that emulsify the brine phase into the oil-exterior phase with a desirable film on the pipe. Such muds need to be conditioned for high electrical stability (ES), and a high mud alkalinity (Pom) to keep the internal phase alkaline. Some earlier treating techniques. Recognizing potential problems, and using proper fluid treatment early during the operation can help avoid corrosion problems. The gypsum muds did not have a high pH necessary for reducing overall corrosion rate and controlling some bacteria. These muds also contained organics that were susceptible to bacteria degradation. And they maintained a sulfate reservoir that sulfate-reducing bacteria species thrived on in anaerobic conditions. Many thousands of wells have been drilled successfully with proper use of quality lignosulfonates and chrome lignites. Proper chemical treatment at the end of the well includes raising the pH, adding a biocide and a dispersible organic amine packer fluid inhibitor if a fresh-water-base fluid is used. We understand that the casing had long, useful life when treated in this manner — while documentation is not easily available, sources indicate this as the trend. Relevant to the subject, Getty Oil Co. wrote a technical paper, published by NACE International, based on South Louisiana field case histories, mainly on high-strength tubulars in lignosulfonate systems. The lignosulfonate was not necessarily deemed the culprit from the field case histories as others had claimed. This operator realized the need for high pH and bacteria control. And the report showed that, with proper conditioning, water-based fluids were more protective than some authors of this period realized. For increased temperature stability of some water-base systems, gypsum and salt muds were treated with a group of new additives called "surfactants," including drilling mud surfactant (DMS) and drilling mud emulsifier (DME). And as usual, during this period, diesel was important for lubrication, inhibition and fluid loss control. Oil was chemically emulsified into the water-based fluids in the 10 – 12% by volume range. The resulting oil-wet film probably played an important part in corrosion control in these systems. These fluids were used in the deep drilling trend in South Texas, with BHTs ranging over 450° to 500°F. THE 1970-1980 PERIOD In the early 1970s, the low-pH KCl / Polymer muds emerged as drilling fluids by choice. These fluids were found to minimize hole problems in many areas, but the external fluid phase could cause corrosion. Salt content is near that of seawater and it was noted that corrosion rates were in the 22 lb / ft2-yr range, and should be monitored with drill pipe corrosion coupons. The contractors quickly realized that the fluids needed to be tested for corrosion rates and treated immediately. The drill pipe corrosion ring coupon was invented during the 1950s, and is still used today, Fig. 1. Corrosion monitoring, testing. During the period discussed in this section, corrosion was monitored with electronic corrosion rate meters, galvanic probes and dissolved O2 meters. Later, it was found that these were not necessary in many cases, as downhole fluid may have a higher corrosion rate than surface measurements would indicate. Some of the factors causing the corrosion included O2 in the fluid, pH and salinity. In addition, dissolved acid gases such as CO2 and H2S were influential in causing severe problems. Scavenger additions for each were made, and corrosion rates were monitored with drill pipe coupons. It was generally found that even a small concentration of sulfide could be less aggressive if O2 content were extremely low. With proper pretreatment, this should mean that there would be fewer washouts and twist-offs to contend with. And less tubular failure or downgrades, meant less rig time. While monitoring with coupons, any mineral scale deposit that showed up could be important to the analyst, as it might hold the key to the corrosion problem source. For instance, if rust were noted, O2 corrosion was suspected. If a chemical reagent containing hydrochloric acid and arsenite is used (iron sulfide test solution), any release of CO2 (fizzing) would indicate carbonate scale presence. This would be caused by the carbonic acid. Using the same reagent, for any sign of a yellow precipitate (arsenic sulfide) that resembled "miniature scrambled eggs," the cause was probably due to hydrosulfuric acid formed by the presence of H2S from some source. Problem / failure analysis. Accurate information on metallurgical failures should be expected from the metallurgical labs. After necessary tests, one could start to draw conclusions as to the cause, and cure (if any), of failures being noted. Working with the contractor and the operator, the nature of the problems, and chemicals or mechanical modifications, could be defined and corrected. In West Texas during 1972, the first chemical proportioning pump was used to force-inject liquid sulfite O2 scavenger into the pump suction line through fittings while drilling. These pumps had found their place in all of the production and petrochemical areas, but somehow drilling personnel had failed to use them. It was found that, with proper use, less chemical was added to achieve desired results. The fluid in the mud pump suction is the only fluid going downhole, and it should be at a low corrosion rate to perform downhole. Corrosion rates were much higher downhole than imagined, compared to surface rates. Research to define this was performed, and dynamic closed loop corrosion rate circulators were designed. This research resulted in an excellent explanation of the drilling systems. When failures occurred, more accurate work was needed and the metallurgical labs were used. This proved that working with both operator and contractor, the problem can be described and corrected; the key is to monitor and act accordingly. Salt muds, additives. With popular KCl / Polymer systems, the preferred treatment continued to be sodium sulfite, preferably with a catalyst added. However, a major problem with limited solubility was discovered, especially in cold weather. The solution also reacted with atmospheric O2 and did not stay active long enough. A few chemical companies designed a product that was more concentrated, 55% to 70%, and that was the birth of the catalyzed ammonium bisulfite base chemical for the drilling industry. This low-pH chemical was also injected into the pump suction pipe to mix with the alkaline fluid, Fig. 2. By theory, O2 was reduced before it had a chance to go downhole and cause much trouble. A companion product was the organic phosphorus-based scale inhibitor. This excellent combination of treating chemicals enjoyed much success; the salty fluids seemed to add to scaling problems, and this treatment aided in eliminating the corrosion cells that could form. Corrosion rate limits. High temperatures, various formations, salts and dissolved gases all contribute to undesirable mud chemistry changes. The corrosion rate of fresh-water drilling fluids, as determined by coupons, should normally be less than 2 lb / ft2-yr, and show no pitting. These coupons are normally left downhole for more than 40 hr — 100 hr is preferred. True control is probably less than 0.5 lb / ft2-yr or less; and it is known that rates up to 100 lb / ft2-yr have been recorded. It is also known that such high rates are not widely discussed; i.e., with rates this high, it is assumed that no one was watching. Rates in some earlier brines have been in the range of 30 lb / ft2-yr, when the drill pipe corrosion coupons were first employed; even this is more than one hundred times too high for good corrosion control. A rate of 2 lb / ft2-yr, or less, in fresh water is generally accepted. Inert gas additive. An inert gas, nitrogen (N2), can be bubbled into open suction pits to reduce and dispel dissolved O2 — there was a patent covering this novel approach. With dissolved O2 very low, it is reasonable to assume that the chemical scavenger addition should be less, and this was demonstrated to be the case. Many of the systems developed were very large in size, and it appears now that it may have taken more personnel to manage them than could be justified in the 1980s economy. Case histories. In Europe (Scotland), a salty drilling fluid was causing a higher corrosion rate than desired; and a decision was made to add dispersible inhibitor. With initial addition, the response was positive — corrosion rate was reduced and the drill pipe's surface condition improved. When salt content approaches 19,000 mg/l Cl, inhibitors tend to be less dispersible. In the Eastern area, a low-pH KCl system in a very cold area showed high corrosion rates. When pH was raised to 12, overall corrosion rate was reduced; and after adding O2 scavenger and scale inhibitor, pitting was eliminated and corrosion rate was controlled. In West Texas, a system was too corrosive for the operator; inhibitors were added. Rates were reduced to a satisfactory range. Testing of an organic inhibitor containing phosphorus showed that desirable rates could be achieved without chromate addition. Synthetic mud evolution. Mineral oils with reduced aromatic content were used to help with environmental concerns. These oil muds were very useful in expanding the choices for drilling. Next came the excitement and success of a new class of muds called synthetic muds. Similar in preparation to the oil muds, they approach the ultimate in dealing with environmental concerns. The external phase is classified as "synthetic" which is cleaner than the mineral oil systems. These new muds show the same trend of corrosion protection as the oil muds. There were no surprises with this successful family of systems, originally using poly alpha olefins as the external phase. INTO THE '90s AND BEYOND Even in the late 1990s, the economy does not totally support all of the means for preventing drill pipe and casing string corrosion damage. Proper tubular care can be time consuming, but ultimately profitable — usually, life is measured by the number of feet of hole that it can drill and be usable. Handling tubulars and sample specimens. When tubulars are delivered as premium pipe, they meet strict standards and will be serviceable. When threads have just been cut, as in a new string, there will be sharp edges that need to be burnished through careful make-up / break-out. This usually is not a problem, but a joint can be ruined on the first run if it is not handled according to recommended practice. API, NACE International and others have technical documents for helping prevent corrosion. In the case of a failed tubular being readied for shipment to the metallurgical lab, carefully wrap each section separately. Try not to let the fracture surfaces touch one another. If they do touch, the metallography work may be impaired. And do not use a torch close to the fracture, as the heat can affect the metal structure. Failures can be very complicated and the lab must be given sufficient bench time. Carefully dry the parts, then wrap them securely for shipment. Do not put pipe dope or oil on the pipe — if the metal is dry, it should not rust or corrode. Do not scrape off any scale, as it can help in identifying the cause. This same technique is approved by API for drill pipe coupons. Dry the coupon and do not apply oil or grease before sending for analysis. For good case histories, quality photographs may be ordered with the initial work order. If the metallurgy cannot be changed to lower the corrosion rate for the intended environment, a corrosion inhibitor film should be considered; and the environment itself can sometimes be changed. When washouts occur due to corrosion fatigue, it is usually a good move to monitor and lower the corrosion rate. The pipe will usually fail after many cycles, but if corrosion rate is kept in an excellent range, it should last much longer. Modern oxygen inhibition. Today, some O2 scavengers are being used with scale inhibitors. The zinc-containing scavengers are still used for treating systems for sulfide control. High pH is also used for treating carbonic acid in some cases; and filming inhibitors still find usage. The most popular type of drilling inhibitor today is the organic phosphate ester-base corrosion inhibitor. These organic phosphorus-containing chemicals that replaced chromates are proving to be less troublesome to use. They do not require a proportioning pump for addition or "tourly" treatments. The inhibitor probably should be classified as an O2 inhibitor, and not an O2 scavenger. They are not recommended for packer fluids, but nearly every water-based drilling fluid can utilize them effectively. CONCLUSIONS Remember that drilling corrosion control can fall in at least three categories: 1) change of chemistry of the drilling fluid liquid phase that contacts the pipe; 2) changing the pipe surface itself with different metallurgy; and 3) use of an inhibitor film to isolate the fluid from the steel surface. The relevant situation can be further defined by "location," i.e.: A) while the pipe is in the hole drilling; B) when pipe is pulled into the derrick and is in contact with air / humidity; and C) while tubulars are stored and stacked on the rack for long periods of time. Each will require a different approach or chemical product to address the problem. Newer and more-powerful inorganic inhibitors for extreme 500°F environments may need to be developed. It is possible that a drilling environment some day may be so aggressive that these different approaches may be needed. This would cover the geothermal area. And certainly, more development in corrosion resistant alloy (CRA) type metallurgy will be needed. This could include special metallurgy such as multi-phase high alloys like MP-35N, Alloy 20, or some Titanium alloy, to be able to have no metallurgical failures due to corrosion. Even the application of the special clad metals or ceramic coatings to isolate a susceptible metal from a hostile environment may be tried. Until this type of action can be justified in the late-1990s economic situation, we need to rely on many more persons that are "aware" of the problem, and also have the follow-through to try various means of reducing corrosion when it occurs. In the last 40 years, we have gone from some fluids being uncontrolled, to a level of possible control. Remember to be aware of potential corrosion problems. Try to seek out the assistance of skilled people with the operator, drilling contractor and fluid service company. Working as a team will determine the best treatments for performance on each well.
The author Thomas E. (Tom) Cox, Senior Research Scientist, M-I L.L.C., Houston, earned a BS degree in chemistry from Stephen F. Austin University, Nacogdoches, Texas, in 1952, and an MS in organic chemistry from Texas A&M University in 1955. He joined Magcobar in 1956, and moved up through various field and research positions to technical coordinator in 1986, when M-I L.L.C., was formed in a joint venture. He has been in his present position since 1991. Mr. Cox is a member of NACE International, and serves as Corrosion Control in Drilling Fluids group chairman. He also is a member of ASM and ACS. He is an active speaker, he has made numerous educational presentations, and he has published several papers / articles on the subject of corrosion. Copyright © 1999 World
Oil |