DUY NGUYEN and NICHOLAS SADEGHI, Nalco
Water soluble demulsifiers and oil soluble demulsifiers were used to treat the emulsion resulting from a CEOR (chemical enhanced oil recovery) project. The demulsifier greatly lowered water content in the oil phase–BS&W (basic sediment and water) <0.5%–and oil concentration in the water phase (less than 50 ppm). Application of this novel demulsifier resulted in a much more effective oil/water separation, with the production of dry oil and clean water at a pilot ASP (alkali, surfactant and polymer) flood that was experiencing very stable emulsions. The demulsification mechanism was also investigated in terms of elastic modulus, particle size and interfacial tension.
This article presents factors associated with CEOR, such as key issues (e.g., emulsions), challenges (e.g., water treatment) and best practices (e.g., a combination of equipment design and demulsifier to treat emulsions). During the application of CEOR floods, breakthrough of the injection chemicals, such as surfactant and polymer or polymer alone, periodically occurs, resulting in stable emulsions. This article was compiled from the literature to report the effects of alkali, polymers, surfactants, asphaltenes, resins and shear rates on emulsion stability.
When treated with polymer alone, the produced fluid was not very stable and resolved into two phases—oil and water. However, the water exhibited a high oily content and was difficult to treat, due to the adsorption of polymer onto the surface of the oil droplets. Zeta potential measurements indicated that oil droplets were not only stabilized by steric stabilization of the polymer but also by electrostatic stabilization. The effect of polymer on emulsion stability in SP (surfactant and polymer) or ASP (alkali, surfactant and polymer) floodings is complicated. Polymer can form a “bridge” between two oil droplets and decrease the emulsion stability. However, polymer can also enhance the emulsion stability via electrostatic and steric stabilization.
Asphaltenes and resins present in the crude oil form a rigid film around water droplets, contributing to high BS&W values. Surfactants and alkali decrease the interfacial tension and zeta potential, contributing to the stability of oil droplets. As concentrations of the injection chemicals in the produced fluid varied, the stability of the emulsion also changed. As a result, the selected demulsifier has to be robust.
BACKGROUND
Primary and secondary recovery techniques, together, are able to recover only about 35% to 50% of oil in place. Since there is a significant amount of oil remaining in the reservoir after primary and secondary processes have been utilized, chemical flooding (using surfactants, polymers and sometimes alkali) has been one of the technologies applied to recover up to an additional 35%. It is estimated that several hundred CEOR field trials have been conducted over the past 50 years, with many occurring during the 1970s and 1980s.1
Surfactant/polymer (SP) and alkali/surfactant/polymer (ASP) floodings today have been re-invigorated by the introduction of more cost-effective surfactants and polymers coupled with improved reservoir modeling. Globally, it is estimated that there were over 20 CEOR projects in field trials or commercial stages in 2011. Additionally, the recent (April 2011) $100/bbl crude price environment continued to stimulate interest in new projects by super-majors, national oil companies and independents.
Emulsions created by chemical flooding have been extremely difficult to break, due to the high concentration of alkali, surfactant and polymer tightly bound with the oil and water. In an earlier article,2 the authors investigated the effects of salinity, temperature, nature of oil and surfactant on emulsion stability, with alkyltrimethyl ammonium bromide as a demulsifier. This article summarizes the effects of alkali, polymers, surfactants, asphaltenes, resins and shear rates on emulsion stability. Also compared is the demulsification efficiency between alkyltrimethylammonium bromide and alkyldimethylbenzyl ammonium bromide, and particle size distribution. The demulsification mechanism was also investigated in terms of elastic modulus, particle size and interfacial tension.
EXPERIMENTAL METHODS
Materials. Pure cationic surfactants of the types, alkyltrimethylammonium bromide (C6-C18TAB) and alkyldimethylbenzylammonium bromide (C10-C18), were obtained from Sigma-Aldrich. The crude oils (SP and ASP) were from the U.S., and their physical and chemical properties are listed in Table 1. The polymer (partially hydrolyzed polyacrylamide, HPAM) from SNF Company has a molecular weight of 8×106 and 30% hydrolysis. Alcohol propoxylate sulfate and alkyl sulfonate surfactants were obtained from Stepan Company. Brine solutions were prepared using various inorganic salts obtained from Sigma-Aldrich. Diethylene glycol monobutyl ether (DGBE) and iso-butanol co-solvents were purchased from Aldrich Chemical.
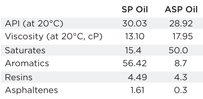
| Table 1. Crude oil physical properties |
|
|
Emulsion preparation. The emulsion (100 ml) was produced in the lab by mixing produced water (i.e., brine solution) containing polymer and surfactants with the oil by shaking the six-ounce prescription bottle mechanically for 10 min., using a heavy-duty, two-speed Eberbach shaker. The demulsifier was added to the above emulsion, and the bottle was again shaken for another 3 min. The amounts (ppm) of demulsifiers added were based on the volume of the emulsion. The ratios of produced water-to-oil used were varied from 1:1 to 9:1.
Focused beam reflectance measurement (FBRM). The FBRM probe is from Mettler-Toledo Lasentec and consists of a 3-MW laser with a wavelength of 791.9 nm that is transmitted through fiber-optics to the probe tip. When the probe is immersed into an emulsion, the laser emitted is reflected, if it scans across the surface of a droplet. The probe measures the reflectance time and calculates the chord length by the product of the time and laser scan speed. A chord length is defined as the straight line distance from one edge of a droplet to another edge. Droplets between 0.5 microns and 3 mm can be measured.
Interfacial tension. The equilibrium interfacial tensions of brine and crude oil after phase separation were measured at 22°C, using a University of Texas spinning drop tensiometer. Aliquots of oil and water were withdrawn with a 25-ml syringe, equipped with a 2-in. needle. Each aqueous phase was brought to temperature in the instrument as the continuous phase. Then, a 1.0-microliter drop of oil phase was introduced as the drop phase, and the system was spun at variable speeds.
Elastic modulus. A Teclis TRACKER tensiometer (Kruss Company) was used to measure the elasticity at 25°C by the pendant and oscillating drop method in controlled surface area mode. An oil droplet was formed and maintained vertically at the end of an inverted needle in a transparent cell containing the aqueous phase (brine solution with demulsifier, polymer and surfactants). The drop was oscillated at different frequencies after the equilibrium interfacial tension was reached and elastic modulus was measured from a change in interfacial tension, and a change in surface area of the oil droplet, due to expansion and contraction. Details about the technique can be found elsewhere.3
Demulsification tests. Demulsification tests were conducted in graduated, 6-oz. prescription bottles to allow for rapid water drop readings. All bottles used 100 ml of emulsion. After pouring the emulsion followed by chemical addition, the bottles were allowed to reach the separator desired temperature via a water bath. Upon reaching the desired temperature, samples were shaken via a mechanical shaker and then returned to the water bath. Water drop readings were recorded in milliliters as a function of time. Water drop values also were used to gauge emulsion stability, where a faster water drop indicated lower emulsion stability. Water samples were taken from the bottoms of the bottles, using a syringe, and oil concentrations in water were deduced from the turbidity measurements.
Following the oil drop readings, the resolved or partially resolved oil from each bottle was analyzed for water content. Using a syringe with a needle, a small portion of oil (about 6 ml) was withdrawn. The syringe tip was set to 10 to 15 ml above the theoretical oil-water interface, as determined by the slug grindout value. This aliquot of oil was added to a graduated API centrifuge tube containing an equal volume of an aromatic solvent, and contents were shaken by hand. The centrifuge tubes were then centrifuged on high speed for three minutes.
Following centrifugation, the percent residual emulsion, typically referred to as BS&W, were noted for each bottle. After recording BS&W values, alkyl sulfonate surfactant (a chemical known to resolve the remaining emulsion) was added to the centrifuge tube. Such chemicals are generally called “slugging or knockout chemicals” and are typically low-molecular-weight, sulfonate-based materials. After slugging, the tube was again shaken and centrifuged as previously described. The BS was, therefore, completely eliminated, and only water remained in the bottom part of the tube. The slug grindout number is reported as a percentage. Smaller values of BS&W and slug indicate drier oil. The time (in min.) elapsed for the total volume of water to separate is taken as a measurement of the emulsion stability
RESULTS AND DISCUSSION
Characterization of oil: As shown in Table 1, physical and chemical properties of the oils were measured and characterized. The API gravity and viscosity were measured at ambient temperature. Saturates, asphaltenes, resins and aromatics (SARA) analysis was performed to identify the total saturates, aromatics, resins and asphaltenes. Resins and asphaltenes are known to stabilize the emulsion.
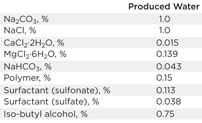
Characterization of produced water: As shown in Tables 2 and 3, concentrations of surfactants and polymer in the produced water from SP and ASP floodings are above 1,000 ppm. Surfactants and polymer were mainly responsible for the stability of oil droplets. Polymer increased the interfacial film between water and oil, decreased the zeta potential (more negative), and blocked aggregation and flocculation of oil droplets. Surfactants decreased the interfacial tension (IFT) and zeta potential, making oil droplets difficult to approach and coalesce. Alkali, such as sodium carbonate, reacted with acidic components of the crude oil and converted them into natural surfactants, which can further stabilize the emulsion.
| Table 2. Surfactant/polymer flooding, produced water formulations |
|

|
| Table 3. Alkali/surfactant/polymer concentrations in produced water from ASP flooding |
|
|
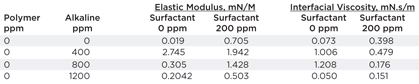
Effect of alkali: W. Di, et al.,4 show that oil-water interfacial elasticity modulus and viscosity both increase, reach maximum, and then decrease with alkaline concentration, Table 4. It correlates with emulsion stability. Alkaline reacts with asphaltenes and resins present in crude oil to produce anionic surfactants. As a result, at low content, alkaline increases the electrical repulsion between oil droplets, oil-water interfacial elastic modulus and viscosity, hence, retarding oil droplet coalescence. However, at high content, alkaline reduces the density of negative charge on an oil droplet surface, due to high ionic strength, decreases oil-water interfacial elastic modulus and viscosity, promoting oil droplet coalescence.
| Table 4. Effect of alkaline on oil-water interfacial rheology4 |
|
|
Effect of SARA on emulsion stability. M. Li, et al.,5 show that unstable emulsions were formed between distilled water and the asphaltene model oil. However, in the presence of 1.2% Na2CO3, the emulsion was very stable because Na2CO3 reacts with asphaltene to form surfactants. In contrast, the saturated, aromatic and resin model oils were not able to form stable emulsions, indicating that the asphaltene is responsible for the emulsion when Na2CO3 is present. This result is in agreement with a high value of interfacial shear viscosity of the asphaltene model oil and the alkaline solution.
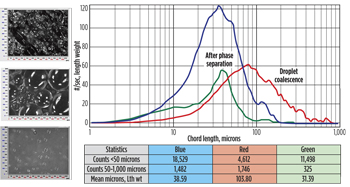
Oil droplet size distribution. Figure 1 shows that oil droplets with chord lengths <50 microns, and 50 to 1,000 microns have nearly constant counts between 5 and 25 min., indicating that the emulsion is stable and droplets do not change over time for the untreated surfactant and polymer (SP) emulsion. When the shear rate is decreased, an increase in the mean oil droplet chord length is observed (from 69.32 microns to 85.36 microns) with a decrease of droplet distribution < 50 microns (from 19,462 counts to 13,708 counts). This suggests that a decrease in shear rate promotes droplet coalescence.
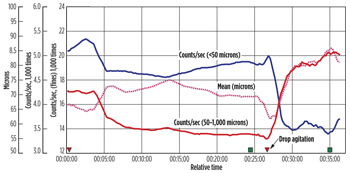 |
| Fig. 1. Particle size distribution of the untreated SP emulsion. |
|
When treated with 200 ppm octyltrimethylammonium bromide (C8TAB), mean droplet size increases from 38.59 microns (initial) to 103.80 microns (red curve), indicating rapid droplet coalescence. Particle counts <50 microns range also decreased by an order of magnitude, Fig. 2. Following coalescence, the oil forms a separate phase, and, as a result, there is significant reduction in mean size (from 103.80 microns to 31.39 microns; green curve) and droplet population. Figure 3 summarizes results of the untreated and treated SP emulsion. The latter showed a decrease in both droplet number and dimension. FBRM can be used as a quantitative tool to rapidly screen chemicals designed for oil-water separation.
 |
| Fig. 2. Particle size distribution of the SP emulsion treated with 200-ppm C8TAB. |
|
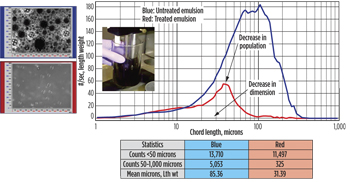 |
| Fig. 3. Comparison of particle size distribution between the untreated and treated SP emulsion. |
|
Elastic Modulus: Elastic modulus, E, is defined as the ratio of surface stress to strain per unit area:
E = dγ/dA/A = dγ/dlnA (dyne/cm) (1)
Elastic modulus measures the resistance of the film to deformation or coalescence. The smaller the value of E, the less stable the emulsion. Elastic moduli for various demulsifiers are listed in Table 5. As can be seen, low interfacial elasticity can reduce the oil concentration in the water phase and BS&W. As the ion-pairs of demulsifier and surfactant adsorb and replace the surfactant at the interface, interfacial elasticity starts to decrease. A similar trend was also observed by Kang.6
| Table 5. Properties of demulsifiers in SP emulsions |
|
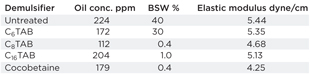
|
Interfacial tension. Interfacial tension measurements have been investigated by several authors to give insights into the mechanisms of demulsifier adsorption at the oil-water interface.7,8 Figure 4 shows the effect of equilibrium interfacial tension on emulsion stability for C14TAB and C18TAB at various concentrations. It can be seen that a good separation with good water quality and low BS&W was observed for the system that exhibited lowest equilibrium interfacial tension. This finding is consistent with the interpretation of the optimum demulsification efficiency, in which a minimum stability is achieved when the demulsifier adsorbed at the interface exhibits the same affinity for the oil and water phases. Typically, the interfacial tension values for microemulsion-water interfaces range from 0.01 to 0.0001 dyne/cm,9 which are much lower than that measured for C18TAB (0.26dyne/cm) at 100 ppm. Perhaps due to the low concentration of demulsifiers used in these experiments (200 ppm), a middle (microemulsion) layer was not observed. Addition of C18TAB and C14TAB beyond their optimum (>100 ppm) resulted in an increase of the emulsion stability and corresponding increase in interfacial tension, perhaps due to the formation of an elastic, viscous film caused by the excess of demulsifier.
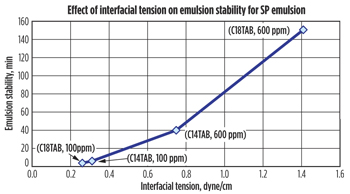 |
| Fig. 4. Effect of interfacial tension on emulsion stability for SP emulsion. |
|
Effect of polymer. M. Li, et al.,5 investigated the effect of polymer on zeta potential, interfacial tension, emulsion stability and interfacial shear viscosity between oil and HPAM solution (i.e., in the absence of surfactant and alkali). Results show that the interfacial tension was not affected by HPAM, suggesting that HPAM is not a surface active agent. However, HPAM is able to adsorb at the interface between the oil and water, as indicated by an increase in interfacial shear viscosity and absolute value of zeta potential. As a result, emulsion stability increased. This proved that oil droplets were not only stabilized by steric stabilization of the polymer, but also by electrostatic stabilization.
Also studied was the effect of polymer on emulsion stability at a fixed surfactant concentration, Fig. 5. When the polymer concentration is above 500 ppm, the polymer adsorbs at the interface and increases the strength of the interfacial film, resulting in enhancing the emulsion stability. However, at lower concentrations, the polymer can also flocculate the oil droplet and decrease emulsion stability through polymer bridging action across oil droplets. This observation is in agreement with results from other authors.4,5 Interestingly, the emulsion was most stable in the absence of polymer, Fig. 5.
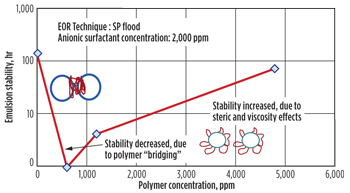 |
| Fig. 5. Effect of polymer on emulsion stability. |
|
Effect of surfactant. Figure 6 shows the effect of anionic surfactant on demulsifier concentration. As can be seen, the amount of demulsifier required to separate the emulsion increased linearly with surfactant concentration. For example, if surfactant concentration is increased two-fold, the amount of demulsifier needed is also increased two-fold.
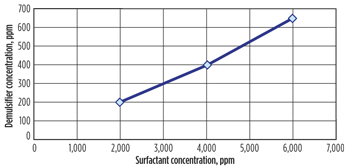 |
| Fig. 6. Effect of surfactant on demulsifier performance. Polymer concentration = 1,200 ppm. |
|
Effect of demulsifier concentration. It is observed that emulsion stability decreased with an increase in demulsifier concentration, Fig. 7. However, addition of a demulsifier above its optimum concentration resulted in an increase of emulsion stability, perhaps due to the formation of an elastic, viscous film caused by the excess of demulsifier. Demulsifier with a longer carbon chain length was more effective.
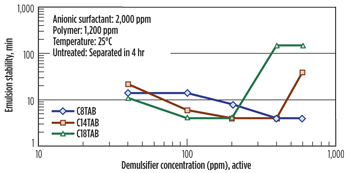 |
| Fig. 7. Effect of demulsifier concentration on emulsion stability. |
|
Comparison of alkyltrimethyl ammonium bromide and alkylbenzyldimethyl ammonium bromide. Figure 8 shows the effect of carbon chain length of alkyltrimethyl ammonium bromide (C6-C18 TAB) and alkylbenzyldimehtyl ammonium bromide (C10-C18 BAB) on demulsification efficiency. The optimum chain length was C8 for alkyltrimethyl ammonium bromide, resulting in BS&W of 0.8% and clean water. For alkylbenzyldimethyl ammonium bromide, optimum chain length was C12. It lowered the BS &W to 0.8% but water quality was much worse than C8TAB.
Challenges and best practices. Table 6 is a summary of challenges and best practices from a roundtable discussion at the SPE EOR conference, held in Kuala Lumpur, Malaysia, July 19-21, 2011. The primary key issues are water/oil quality, equipment design and demulsifier selection, especially for offshore applications, since the retention time is short and space is limited. One of the best practices is to simulate the emulsion in the lab and identify the best demulsifier.
| Table 6. Key issues and challenges of chemical EOR |
|

|
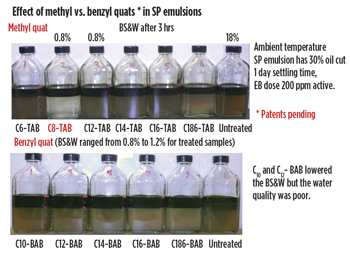 |
| Fig. 8. Comparison of alkyltrimethylammonium bromides with alkyldimethylbenzyl ammonium bromide in SP emulsion |
|
CONCLUSIONS
The asphaltene fraction dominates the emulsion of a crude oil and Na2CO3 solution. The effect of polymer on emulsion stability in SP (surfactant and polymer) or ASP (alkali, surfactant, and polymer) floodings is complicated. Polymer can form a “bridge” between two oil droplets and decrease the emulsion stability; however, polymer can also enhance the emulsion stability via electrostatic and steric stabilization. Ion pairs formed between the cationic demulsifiers (patents pending) and anionic surfactants may explain for the accelerated separation of oil-in-water emulsions with good oil and water quality during chemical EOR processes.
At the optimum demulsification efficiency, interfacial tension is lowest, suggesting that ion-pairs exhibit the same affinity for both phases. The demulsification mechanism is proposed that the demulsifiers partially replace surfactants adsorbed at the oil-water interface, thereby causing a decrease in elastic modulus and interfacial tension. Alkyltrimethylammonium bromides are more effective than alkyldimethylbenzyl ammonium bromide at separating the oil-in-water emulsion stabilized by chemical EOR. 
LITERATURE CITED
1. Thomas, S., “Chemical EOR: The past—Does it have a future?”, SPE distinguished lecture, 2005
2. Nguyen, D., N. Sadeghi and C. Houston,”Emulsion characteristics and novel demulsifiers for treating chemical induced emulsions”, SPE paper 143987, SPE Enhanced Oil Recovery Conference Kuala Lumper, Malaysia, July 19-21, 2011.
3. Puff, N., A. Cagna and R. Douillard, “Surface dilational rheology of proteins adsorbed at air/water and oil/water interfaces,” J. Colloid Interface Sci., v.208, p. 405-414, 1998.
4. Di, W., M. Xiangchun, Z. Ruiquan, C. Yan, W. Qingsheng, and L. Huicheng, “Emulsification and stabilization of ASP flooding produced liquid,” SPE paper 65390, SPE International Symposium on Oil Field Chemistry, Houston, Feb. 13-16, 2001.
5. Li, M., J. Guo, B. Peng, M. Lin, Z. Dong and Z. Wu, “Formation of crude oil emulsions in chemical flooding,” Emulsions and Emulsion Stability, Second Edition, edited by Johan Sjoblom, Surfacant Science Series, Vol. 132, CRC press.
6. Kang, W. L., “Study on the interaction mechanism of chemicals used in ASP flooding in Daqing oilfield,” Ph.D dissertation, College of Daqing Petroleum, China, 2000.
7. Goldszal, A., and M. Bourrel, “Demulsification of crude oil emulsions: Correlation to microemulsion phase behavior,” Ind. Eng. Chem. Res., v. 39, p.2746-2752, 2000.
8. Breen, P.J., “Adsorption kinetics of demulsifiers to an expanded oil-water interface,” ACS Symp. Ser., v. 615, p. 268, 1995.
9. Salager, J.L., “Guidelines for the formulation, composition and stirring to attain desired emulsion properties.” In Surfactants in solution; Chattopadhyay, A.K., K. L. Mittal, Eds.; Surfactant Science Series 64; Marcel Dekker; New York, 1996.
ACKNOWLEDGEMENTS
The authors thank Nalco for permission to publish this work, and express their sincere gratitude to Professors George Hirasaki and Clarence Miller at Rice University.
|
The authors
|
| DR. DUY NGUYEN is a research associate at Nalco Company. He graduated with a BS degree in chemical engineering and a PhD in surface & colloid chemistry in the U.S. He has received 25 U.S. patents, published over 15 scientific publications, and presented papers at numerous conferences. His research interests center on well stimulation, foams, hydraulic fracturing, enhanced oil recovery, emulsions and microemulsions. |
|
| NICHOLAS SADEGHI is a chemist at Nalco Co. He earned a BS degree in Biochemical and Biophysical Sciences, with a focus on chemistry, from University of Houston in 2007. His experience lies in oilfield chemicals, EOR and demulsifiers, specializing in paraffin and asphaltene mitigation. |
|
















