STAN McCOOL, University of Kansas; and GINNY WEYLAND, National Energy Technology Laboratory
As part of the US government’s Oil and Natural Gas Program, the University of Kansas is conducting research to enable independent oil producers to accurately assess whether oil production from their reservoirs can be enhanced by next-generation chemical flooding. The Department of Energy program is managed through the National Energy Technology Laboratory (NETL). After classifying Kansas reservoirs amenable to flooding, selection criteria were established to identify the best candidates for chemical flooding. Crude oil samples were collected, and laboratory work was performed to design chemical formulations for specific oils/reservoirs. Finally, reservoir simulations were conducted to determine the expected response to chemical flooding in the selected fields. Future work includes evaluating the economics of field applications.
SELECTING RESERVOIRS
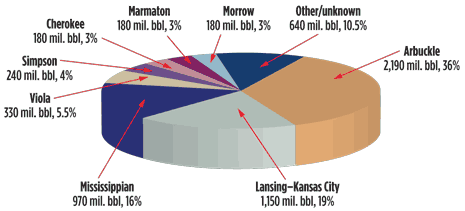
Kansas reservoirs were classified using geological characteristics such as lithology, trap and drive mechanisms. Together, the Arbuckle group and Mississippian reservoirs have provided 52% of the oil production in Kansas, Fig. 1. However, the research team found that neither of these is amenable to chemical flooding due to natural water drives from underlying aquifers.
On conducting interviews with several of the state’s largest independent producers, researchers discovered that the frequency of waterflooding in a specific rock unit was the best indicator of favorable characteristics for chemical flooding. Commonly waterflooded formations in central and western Kansas include the Missourian Lansing and Kansas City groups, the Pennsylvanian Marmaton, Cherokee and Marrow groups, the Mississippian Chester group and the Ordovician Simpson group.
Since chemical flooding is a slug-type process, whereby residual oil is mobilized and displaced to production wells by the chemical slug moving through the reservoir, maintaining the integrity of the slug is crucial. Efficient sweep of the reservoir during a waterflood suggests that the fluid-flow characteristics of the reservoir are sufficient to maintain slug integrity. Therefore, efficient sweep is considered an indicator that a chemical flooding application will be successful.
As detailed fluid-flow characteristics have not typically been determined for most mature Kansas reservoirs, other selection criteria must be employed to identify reservoirs that are the best candidates for chemical flooding: oil recovery response, waterflood age and well status, and evidence of flood containment.
During waterflooding, efficient volumetric sweep is indicated by a significant and sustained oil recovery response. Such sweep efficiency within a reservoir points to it being a strong candidate for chemical flooding. Under the second criterion, a reservoir that has a very mature waterflood, where the majority of wells have already been plugged, is not a strong candidate for chemical flooding.
To be successful, a chemical flood must be contained within a specified pattern area. Boundaries must be clearly defined, and there should be no evidence of communication between multiple zones in a reservoir.
A database of roughly 5,800 oil reservoirs in Kansas was constructed from publically available data (now available online at www.torp.ku.edu). However, the uncertainty of historical production data due to misallocation of the oil produced in a field to various producing zones made the use of the database alone as a selection tool problematic. The research team found that contacting Kansas oil producers to discuss potential candidate reservoirs was much more fruitful. Ten leases were chosen for further study. The producing formations in the fields that were selected include the Lansing-Kansas City, Marmaton, Cherokee, Morrow, Chester and Simpson.
 |
|
Fig. 1. Approximate oil production in Kansas from 1889 to 2002 by stratigraphic unit. Image courtesy of the Kansas Geological Survey.
|
|
IDENTIFYING EFFICIENT CHEMICAL SYSTEMS
Chemical flooding encompasses the use of surfactant (S), polymer (P) and alkali (A) chemicals. Different combinations are required for different applications, and the processes are therefore often referred to, for example, as ASP or SP flooding, to signify the types of chemical used. Polymers are added to increase viscosity of the flooding fluid, which can improve the sweep of the reservoir volume, referred to as mobility control. Alkali is used for acidic oils that are reactive with alkali to produce soaps (surfactants) in the reservoir and to enhance the performance of surfactants. Surfactants are employed to reduce the interfacial tension between the injected fluid and the oil so that trapped oil can be mobilized and produced, providing a clean sweep of the contacted reservoir rock.
Several choices are available for polymers and alkali selection, while there are many options for surfactants. Surfactant systems are usually a combination of two or more surfactants with the addition of co-solvents and other chemicals to enhance their performance.
The process employed to design efficient chemical systems containing surfactants is based on phase behavior studies where different chemical formulations are mixed with the crude oil and then allowed to equilibrate at the reservoir temperature. Systems that form three-phase microemulsions with appropriate characteristics are sought. These characteristics include:
• A microemulsion phase that coalesces and equilibrates in less than seven days
• A relatively large volume of the middle-phase microemulsion, as measured by the solubility parameter
• Absence of viscous phases and macroemulsions
• A one-phase, clear, homogenous mixture as the aqueous surfactant mixture, at both surface and reservoir temperatures.
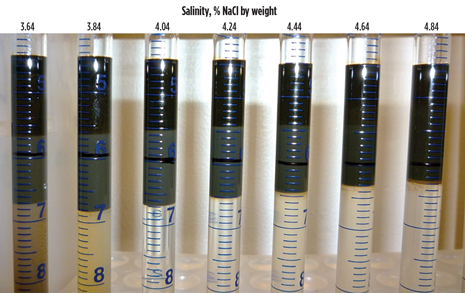
Phase behavior is often studied using salinity scans, in which a chemical system (surfactants and co-solvents) is prepared in a series of solutions where only the salinity is varied. The solutions are contained in disposable pipettes and are mixed with the crude oil being tested. The pipettes are sealed, shaken and then allowed to equilibrate in an oven at reservoir temperature. The tubes are visually inspected periodically to measure phase volumes and then tipped to determine fluidity of the phases.
An example of a salinity scan is shown in Fig. 2 for a surfactant system and crude oil from one of the selected leases. Three liquid phases are observed in each tube: an aqueous lower phase, a middle-phase microemulsion and an upper oil phase. The level of the aqueous chemical formulation in a tube before addition of crude oil is marked by the black line on the tube. The middle phase microemulsion is composed of surfactant chemicals and solubilized oil and water. The volumes of solubilized oil and water in the microemulsion are the volumes of microemulsion above and below the black line, respectively. The optimal salinity occurs when these volumes are equal, which can be seen in Fig. 2 to occur between 4.04% and 4.24% NaCl (third and fourth tubes). Interfacial tensions between the liquid phases are very low at this condition. If the ratios of the solubilized volumes of oil and water to the volume of surfactant in the system—termed a solubilization parameter—are large, then the aqueous system provides excellent performance as an oil recovery fluid in flow tests through rock material.
| TABLE 1. Phase behaviors of efficient formulations for several crude oils |
 |
Chemical selection for a particular application can require considerable laboratory work. The formulation for the chemical system in Fig. 2 contains the primary surfactant Petrostep S1; a co-surfactant, Petrostep S2; a co-solvent, sec-butyl alcohol; and sodium carbonate (Na2CO3) as an alkali. To arrive at the formulation, several surfactants and co-solvents were tested as well as different concentrations and ratios of chemicals. Over 10,000 formulations have been tested for the crude oils from the selected leases.
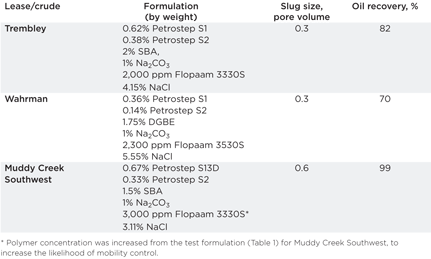
Phase behavior studies have been conducted and chemical systems have been identified that meet the criteria of efficient systems for crude oils from seven of the 10 leases, Table 1. Efficient systems are ones that have solubilization ratios above 10 and for which equilibrium is achieved in seven days or less. Additionally, the optimal salinity will be less than the aqueous stability value. This ensures that the injected aqueous formulation will be a one-phase liquid that does not contain precipitates or a second liquid phase that could separate and jeopardize the formulation.
The optimal salinity will be less than the aqueous stability value to ensure that the injected aqueous formulation does not contain precipitates or a second liquid phase that could separate and jeopardize the formulation.
OIL RECOVERY PERFORMANCE
Oil recovery performance of the identified chemical systems was measured in flow experiments in Berea sandstone cores. Foot-long cores were saturated with brine and oil-flooded. The cores were then waterflooded to establish residual oil saturations of about 35%. Each chemical flood was conducted by injecting a slug of the chemical system followed by a polymer drive. Polymer drives contained the same concentrations of polymer as contained in the chemical slug, or slightly greater. Performance was judged on the percentage of residual oil recovered by the chemical flood.
Performance of the chemical systems for crude oils from the Trembley, Wahrman and Muddy Creek Southwest leases was determined in initial flow tests. The sizes of the chemical slugs in terms of pore volumes of the Berea cores and the amount of oil recovered are given in Table 2. Seventy percent or more of the waterflooded residual oil was mobilized and produced for the three crude oils. Although these results are acceptable, oil recoveries in the 90–100% range were anticipated. Laboratory work is continuing in the effort to increase oil recovery performance by testing additional chemical formulations and by improving the chemical flood design.
 |
|
Fig. 2. Photograph of a salinity scan for the formulation containing (by weight) 0.625% Petrostep S1, 0.375% Petrostep S2, 2% sec-butyl alcohol, 1% Na2CO3 and 2,000-ppm Flopaam 3330S polymer, with Trembley crude oil at 46.1°C.
|
|
| TABLE 2. Oil recovery performance |
 |
CONTINUED RESEARCH
Chemical floods will be designed for two or more of the most promising prospects for the development of a field demonstration project. Selection of leases for designs of field applications will be based on the laboratory results and an analysis of field data. The University of Kansas will develop geological reservoir models and perform engineering analysis of historical injection and production data. Computer simulation will be used to match laboratory flow tests and to predict field-scale processes. Injection and production results from the chemical flooding simulations of the field applications will be used for economic analyses. Capital and operating costs will be estimated.
Principal results of the project will be an assessment of the technical performance and an economic analysis of field applications of chemical flooding. Favorable economics will provide the basis for the development of field applications and the opportunity for the public and independent oil producers to access field results on the performance of chemical flooding processes. 
|
THE AUTHORS
|
 McCOOL@KU.EDU / STAN McCOOL is an engineer with the Tertiary Oil Recovery Project at the University of Kansas, where he has conducted research on chemical recovery processes for 30 years. He holds BS, MS and PhD degrees in chemical engineering from the University of Kansas. McCOOL@KU.EDU / STAN McCOOL is an engineer with the Tertiary Oil Recovery Project at the University of Kansas, where he has conducted research on chemical recovery processes for 30 years. He holds BS, MS and PhD degrees in chemical engineering from the University of Kansas. |
|
 VIRGINIA “GINNY” WEYLAND is a Project Officer for the Strategic Center for Natural Gas and Oil within NETL. She earned a BS degree in geology from Texas A&M University and then worked as a petroleum geologist for Sioux Natural Gas before joining the US Department of Energy, starting as a geologist at the Naval Petroleum Reserve. VIRGINIA “GINNY” WEYLAND is a Project Officer for the Strategic Center for Natural Gas and Oil within NETL. She earned a BS degree in geology from Texas A&M University and then worked as a petroleum geologist for Sioux Natural Gas before joining the US Department of Energy, starting as a geologist at the Naval Petroleum Reserve. |
|





 McCOOL@KU.EDU / STAN McCOOL is an engineer with the Tertiary Oil Recovery Project at the University of Kansas, where he has conducted research on chemical recovery processes for 30 years. He holds BS, MS and PhD degrees in chemical engineering from the University of Kansas.
McCOOL@KU.EDU / STAN McCOOL is an engineer with the Tertiary Oil Recovery Project at the University of Kansas, where he has conducted research on chemical recovery processes for 30 years. He holds BS, MS and PhD degrees in chemical engineering from the University of Kansas.  VIRGINIA “GINNY” WEYLAND is a Project Officer for the Strategic Center for Natural Gas and Oil within NETL. She earned a BS degree in geology from Texas A&M University and then worked as a petroleum geologist for Sioux Natural Gas before joining the US Department of Energy, starting as a geologist at the Naval Petroleum Reserve.
VIRGINIA “GINNY” WEYLAND is a Project Officer for the Strategic Center for Natural Gas and Oil within NETL. She earned a BS degree in geology from Texas A&M University and then worked as a petroleum geologist for Sioux Natural Gas before joining the US Department of Energy, starting as a geologist at the Naval Petroleum Reserve.
