Controlling the effects of geochemical interactions between the shale and the fracturing fluids has helped shale gas operators preserve reservoir communication to the wellbore, reduce sour gas potential and tailor flowback water for reuse.
Matt Blauch, Superior Well Services
Operators exploring for natural gas in shale reservoirs have improved well completions by controlling the effects of geochemical interactions between the shale and the fracturing fluids. Enhancing control over deposits of geochemical precipitates, scale, microbially induced biogeochemical interactions, and high salinity has helped preserve reservoir communication to the wellbore, and has reduced the potential for generation of hydrogen sulfide (sour gas).
Geochemical control also enables operators to tailor frac flowback water so it can be reused in subsequent fracturing operations while minimizing concerns of subsequent formation damage due to geochemical precipitation. Significant reductions in frac fluid expense have been observed from the reuse of flowback, due to the reductions in transportation costs of frac water, purchases of freshwater and water disposal volume.
BACKGROUND
Fracturing shale reservoirs requires the use of large volumes of incompressible fluid (water) to create effective complex fracture surface area. Upon flowback, large volumes of fluid must be managed to minimize impairment of gas production. Initially, during fracturing, chemicals are added to the water to enhance its friction reduction properties during proppant placement at typically high rates and pressures (i.e., 80–100 bbl/min. and 7,000–10,000 psi). The ultimate goal of this process is to provide a long-lived path through which the gas can flow uninterrupted into the wellbore and on to the sales line. Unfortunately, typical shale wells exhibit a steep gas production decline curve.
Along with the introduced additive components, frac fluid in close contact with the shale during the course of the stimulation treatment may, when recovered, contain a wide variety of dissolved constituents such as salts. These constituents can make wastewater disposal difficult and expensive, and can potentially impair gas production by yielding damaging precipitants within the fractures, perforations and wellbore.
Potentially damaging formation products picked up downhole by fracturing fluids are 1) soluble salts; 2) metal ions including iron, barium, strontium, calcium and magnesium; 3) solubilized carbonates such as calcite, dolomite and siderite; 4) solubilized sulfates including gypsum, anhydrite and celistite; 5) scale- and gas-forming microbes; and 6) significant levels of anions including carbonate, bicarbonate, sulfate and chlorides. These byproducts cannot be released without treatment into area streams, waterways and water supplies; therefore, they must be purified at great expense or transported to permitted downhole disposal wells.
A cost-effective alternative approach to maintaining a supply of fracturing fluid is reuse of flowback frac fluid without extensive treatment, a procedure that is made possible by analyzing, performing geochemical modeling upon, and minimally treating the flowback frac fluid. A percentage (10%–70%), but not all of the original frac fluid volume that was pumped downhole returns to the surface before the well is connected to the sales line; therefore, only a small percentage of freshwater is normally required for a new fracturing treatment.1
DEALING WITH HIGH SALINITY
Slickwater pumped at high rates with low sand concentration has been very effective at treating shale formations. An ideal slickwater additive has been found to be a liquid-polymer emulsion that is a salt-tolerant friction reducer (STFR) designed especially for compatibility with the specific shale lithology. Use with brine fluids at NaCl concentrations from 2% to 12% is feasible.2,3 Salt tolerance allows frac fluid of high salt concentration to be reused repeatedly provided that other geochemical properties are controlled.
GEOCHEMICAL AND BIOGEOCHEMICAL DEPOSITS
Three types of scale or geochemical precipitates are of major concern to shale gas producers: carbonate, sulfate and iron-based depositions. In many shale completions, the frac flowback water contains significant levels of ions that can impair production. Unmanaged, this geochemical environment can spawn precipitates within the created fracture network and cause scale to accumulate in perforations, piping and surface equipment. Preventing such accumulations requires analysis of the flowback water to identify and evaluate geochemical deposit-producing potential. An effective treatment method is twofold:
1. Based on the geochemistry analysis, apply an appropriate selection and dose of scale control that is designed not to negatively affect the fluid pH and resultant friction reducer (FR) performance.
2. When necessary, conduct remediation of the water for certain problem species. The ideal treatment should address all scale situations predicted during post-frac flowback; conform to National Pollutant Discharge Elimination System (NPDES) standards; and pass appropriate aquatic toxicity standards if surface disposal is required.
Inclusion of the right blend of inhibitors in the treatment helps prevent formation of certain geochemical deposits by the mechanism of crystal modification. Polyacrylic acid of the proper molecular weight can further prevent the formation of calcium, barium or strontium sulfate scales. Iron control, discussed below, is an important component of the solution since the presence of iron tends to deactivate the effectiveness of conventional scale inhibitors; preventing the formation of iron-based scales has been an industrywide problem.
Sulfate-reducing and iron-metabolizing microbes play an important role in the formation of geochemical precipitates and must therefore be considered in any geochemical discussion. Control measures must be followed to negate the effects of downhole “extremophile” sulfate-reducing microbes and conventional spore-forming bacteria. A 20% active, liquid brominated propionamide, commonly referred to as DBNPA, is an effective choice for biogeochemical control. DBNPA works in the presence of hydrocarbons, begins to kill instantly, and will decontaminate a system within 1 hr. One benefit of DBNPA is its short half-life in the environment. DBNPA is considered one of the safest biocides when used or spilled; it breaks down into the innocuous components bromine, nitrogen and water. The non-foaming biocide is effective against both aerobic and anaerobic microorganisms. It is water-soluble, easy to mix and dilute, and registered with the US Environmental Protection Agency as non-persistent. DBNPA is also not affected by hard water or salts.
IRON CONTROL
An iron-control compound is necessary that chemically maintains the iron in formation fluids in the reduced-valence state and prevents both precipitation and re-precipitation of iron compounds and minerals. The iron-control agent should inhibit the iron from converting to insoluble particulates that can damage fracture conductivity and reduce the production potential of the formation.1 The compound must provide adequate iron control at a low dosage rate without lowering the system pH and, thus, reducing FR performance. The chemical additive should work synergistically with scale-control additives both in preventing precipitation of difficult post-frac geochemical species, such as siderite (which can form in hydraulic fractures), and in enhancing performance of FR chemistry.
Traditional iron-reducing and chelating agents consist of acidic chemicals such as citric acid, acetic acid and ethylenediaminetetraacetic acid (EDTA), which keep the iron in formation in a soluble or reduced form (ferrous iron) through pH reduction and chelation chemistry. This method of iron control performs inconsistently, particularly when the formation acts to neutralize the acid and the acid’s capability to control iron and other metals. Acids or chelants also reduce frac fluid pH and, thus, can adversely affect FR and other chemical performance. Alternative iron control additives should be sought that avoid these drawbacks, thus allowing optimal performance of the FR, scale control agents and biocide.
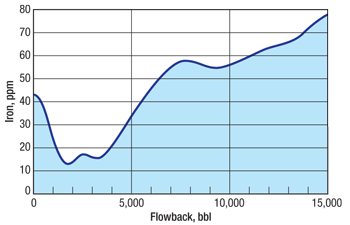
Figure 1 illustrates the need for integrating iron control into the frac fluid, even though the initial water used to frac may contain no iron. Upon contact with the downhole environment, iron and other metal ions are leached and/or released into the frac flowback water. If not controlled, free iron may be available to form a number of iron species that can be damaging to gas production and potentially to the unloading of the frac fluid during well cleanup. For example, geochemical simulation has shown that siderite (FeCO3) can be a problematic iron precipitate.
 |
|
Fig. 1. Iron analysis from a shale well flowback. Presence of iron in the flowback increases as frac fluid is exposed to the formation and picks up iron.
|
|
GEOCHEMICAL SIMULATION
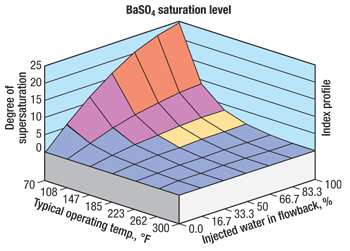
A geochemical simulator may be used to predict the tendency for a wide range of geochemical precipitation from the shale completion flowback fluid. A geochemical simulator shows the tendency for specific mineral precipitates such as barium sulfate and iron carbonate to form, Fig. 2. This process is used to ensure that the appropriate level of protection is being provided by the control chemical.
 |
|
Fig. 2. Geochemical simulation result showing tendency for barite precipitation from a shale well flowback.
|
|
WATER ANALYSIS EXAMPLES
Case histories illustrate the utility of conducting detailed water analyses before preparation of frac fluids for use in a shale reservoir.
Case 1. An operator recovered 15,000 bbl of flowback water, accounting for a load recovery of approximately 40%.4 Analyses were performed on 19 samples; Table 1 presents results from five samples taken throughout the flowback process.
| TABLE 1. Flowback water analysis (Case 1) |
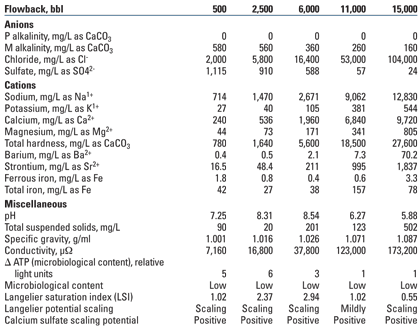 |
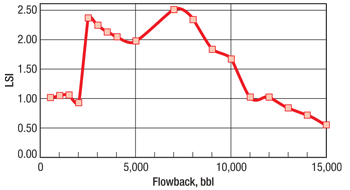
Analysis shows that the volume of dissolved solids increases and pH and alkalinity decrease as flowback progresses. Calcium and sodium are the most prevalent cations. As shown by the Langelier saturation index (LSI) in Fig. 3, the greatest potential for carbonate scale formation occurs when flowback represents 10–30% load recovery. Divalent cations including calcium, magnesium and strontium show dramatic increases as late-stage water is produced to the surface. Decreasing sulfate in the late stages of flowback indicates that sulfate is the limiting ion, and its decrease may be indicative of sulfate precipitation downhole. Solubility of barium sulfate is low, with the potential for creating an aggressive geochemical precipitate or scale. Iron content increases in late stages of flowback, prompting consideration of taking iron control measures.
 |
|
Fig. 3. The Langelier saturation index (LSI) shows high potential for development of carbonate scale (Case 1).
|
|
Based on this analysis, the fluid designed for the ensuing frac treatment was the flowback water along with 1) scale inhibitors designed for multiple sulfate and carbonate species, 2) an iron-control agent with potential to control iron carbonate, and 3) a biocide designed for extremophiles and environmental risk mitigation.
Case 2. A shale gas producer was using surface pond water as a base for mixing frac fluid for a Haynesville Shale gas well. The water was analyzed and run through a friction loop to assess its compatibility with an STFR and compare STFR performance with that of a guar FR. Flowback water was also analyzed and geochemical simulation conducted to determine the propensity for downhole geochemical precipitates and the need for geochemical controls in the fracturing design.
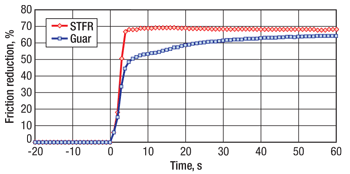
Three friction-loop tests showed that STFR is compatible with the chosen surfactant and other additives. STFR was shown to develop friction reduction more rapidly and efficiently, and achieve a higher FR level, than guar, Fig. 4.
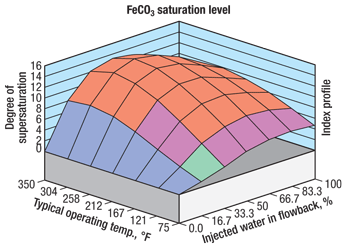
The surface water used is of low alkalinity, hardness, iron and chloride content, indicating that it is an excellent source water for frac fluid; however, geochemical controls are suggested based on modeling of the water equilibrated with the downhole environment. The slightly alkaline pH is well within the operating range of STFR. Although the water contains fine suspended solids that result in high turbidity, it does not impair STFR performance. Geochemical analysis of flowback samples shows a tendency for precipitation of siderite, gypsum and celestite if no controls are applied, Fig. 5.
 |
|
Fig. 4. Comparison of salt-tolerant friction reducer (STFR) to guar FR for development of friction reduction in pond water (Case 2).
|
|
 |
|
Fig. 5. Post-frac flowback water geochemical simulation showing siderite results (Case 2).
|
|
In view of the above analysis, the frac water design included a biocide, a scale inhibitor system, an iron-control agent and a salt-tolerant friction reducer. 
LITERATURE CITED
1 Houston, N. et al., “Fracture-stimulation in the Marcellus Shale: Lessons learned in fluid selection and execution,” SPE 125987 presented at the SPE Eastern Regional Meeting, Charleston, W.V., Sept. 23–25, 2009.
2 Reese, R. R. and P. Rey, “Method of fracturing subterranean formations utilizing emulsions comprising acrylamide copolymers,” US Patent No. 7,482,310 B1, Jan. 27, 2009.
3 Gulf Publishing Company, “Superior Well Services, Inc. wins the ‘Best Drilling, Completions & Production Fluids Award’ for its WFR-38 slickwater system,” press release, Oct. 16, 2009.
4 Blauch, M. E., Myers, R. R., Moore, T. R. and B. A. Lipinski, “Marcellus Shale post-frac flowback waters: Where is all the salt coming from and what are the implications?” SPE 125740 presented at the SPE Eastern Regional Meeting, Sept. 23–25, 2009.
|
THE AUTHORS
|
 |
Matt Blauch is the Director of Product Development at Superior Well Services. He has more than 25 years of industry experience, including 16 years working with unconventional gas, principally coalbed methane and shale gas. Mr. Blauch earned a BS degree in geology from Juniata College, Huntingdon, Pa., in 1982 and an MS degree in geology from the University of Akron.
|
|
|









