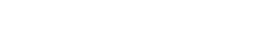Among other findings, the results suggest that deposition occurs mostly at initial contact with equipment surfaces.
Kamran Akbarzadeh, Schlumberger; Zhongxin Huo and George Broze, Shell Projects and Technology
Scientists have been trying to predict the potential for asphaltene precipitation since the early 1800s. What they have collectively determined is that asphaltene is present in most crudes. The principal questions for researchers today are whether or not asphaltene will precipitate, and, if it does, whether it will adhere to production tubulars, Fig. 1.
 |
|
Fig. 1. Asphaltenes can be deposited anywhere in the production system, from the near-wellbore formation to the separator, or even in the pipeline. Courtesy of Oilfield Review, Summer 2007.
|
|
A wide variety of tests has been developed, first to measure the concentration of asphaltene in crude oil samples and then to characterize it so a treatment could be developed to prevent its precipitation and deposition on production tubulars. The most sophisticated of these tests involve mass spectrometry measurements or molecular diffusion measurements. But even these rigorous tests failed to yield a treatment for asphaltenes in a wide variety of crude oils. The problem is that asphaltene itself can exist in widely divergent molecular weights and sizes ranging from 300 g/mol to 1,400 g/mol. Its emergence is generally a function of pressure, but even when asphaltene precipitates, whether or not it will stick to the walls of tubulars is difficult to predict.
The physical conditions affecting crude oil as it makes its way from the reservoir to the pipeline are widely diverse, particularly in deepwater fields. Large variations in pressure and temperature are encountered; mixing of incompatible fluids and gases can destabilize the produced fluid, increasing the risk of asphaltene precipitation and deposition. As a result, scientists have sought ways to mimic field conditions in the laboratory so valid tests could be designed that would conclusively determine the likelihood of precipitation and/or deposition of asphaltene.
DEAD OIL VS. LIVE OIL
Seeking a practical answer to the reliable prediction of the location and extent of asphaltene precipitation and adhesion, Shell Exploration & Production Company and Schlumberger worked to modify Schlumberger’s proprietary RealView test apparatus to simulate real-world flow conditions for live crude oil in a deposition cell, so scalable results could be reasonably extrapolated to fit field conditions. The live-oil solids deposition cell, based on the Taylor-Couette flow principles,1 allows independent variation of test parameters to quantify the effects of pressure, temperature, composition, surface type, flow regime and shear on the deposition behavior of waxes and asphaltenes.
In addition to answering the deposition question, Shell was interested in solving the inhibitor enigma. Previously, the company had observed that certain applications of inhibition chemicals to the flow stream would prevent the adhesion of asphaltene to production tubulars, but then it was discovered that the solution was not universal. Sometimes very small concentrations of inhibitors were required to achieve significant results, but increasing the concentration of inhibitors by a factor of three did not improve efficiency. Also, the efficacy of inhibitors varied from crude to crude, indicating unknown compositional dependence. The high cost of inhibition chemicals warranted a more reliable solution.
Early attempts at finding a solution to asphaltene deposition involved a high-pressure deposition cell. In it, a fixed volume of crude oil was tested by changing its physical environmental parameters in an attempt to generate a reliable phase diagram with a predictable asphaltene precipitation envelope. A variable-speed DC motor rotated an internal spindle that imposed a dynamic shear on the sample similar to that of an active flowline. However, it quickly became apparent that the closed-chamber test could not reliably predict deposition, largely because once asphaltene started to deposit, asphaltene was depleted from the solution. The fixed oil volume often did not contain enough asphaltene to make a significant deposit. A system was needed that more accurately simulated crude oil flow in production tubulars, meaning a virtually inexhaustible supply of fresh live crude oil was needed, Fig. 2.
 |
|
Fig. 2. A comparison of the early batch test apparatus (left) with the improved flow-through device (right) shows dramatic improvement in asphaltene deposition. (Click image to see larger version.)
|
|
BACK TO THE DRAWING BOARD
From the failure of the close-chamber test apparatus, it was determined that, although asphaltene precipitation is necessary for deposition to take place, deposition is not a foregone conclusion. Additional parameters such as flow shear rate, surface type and characteristics, particle size and particle-to-surface interactions influence deposition. A test was needed that could reliably simulate these parameters.
Working together to determine the necessary specifications, the researchers made a change that transformed the previous high-pressure closed-chamber design into a dynamic one. By adding a flow-through feature, a sufficient volume of sample fluid could be introduced into the test cell so that even samples containing low asphaltene concentrations could be tested. The only requirement was that live oil sample volumes had to be 900 cc or larger, as opposed to the 150-cc sample requirement for the closed-chamber, or batch, test. The flow-through feature made the difference. With the cell’s contents continually replenished with sample fluid having the same composition as the initial fluid, the apparatus more realistically mimicked flowline conditions.
PROCEDURE
Starting with the test chamber filled with stock-tank oil and the rotating spindle turned off, the test sample is slowly introduced. The original oil load is displaced out of the cell and discarded when analysis shows that the sample is near 100% test oil composition. Then the spindle is started. Cell pressure is reduced to test pressure, which is about 100 psi above the fluid’s bubble point. For the duration of the test, all parameters—flowrate, pressure and temperature—are regulated carefully. Finally, helium is used to displace the oil at test temperature and pressure. The cell is opened, and the accumulation of asphaltene on the cell walls (analogous to flowline walls) is measured. To ensure that all parameters were controlled for the first test run of the new apparatus, the researchers created a live-oil sample (representative of the downhole fluid) from the parent stock tank oil and recombined it with a synthetic gas for a fixed gas-oil ratio of 865 ft3/bbl.
Once the variables were determined to be fully controlled, the test apparatus was used to conduct 20 tests. The first four tests were batch type, and the final 16 were flow-through tests. Test objectives were clear:
• Compare deposit amounts between batch and flow-through methods.
• Determine the shear effect (spindle rotational speed simulating actual flowline conditions) on deposition tendency.
• Determine the effect of residence time (average time of exposure in the test cell).
• Determine if asphaltene is deposited at conditions above the detected asphaltene onset pressure at reservoir temperature.
• Determine the efficiency of the asphaltene inhibitor being used.
RESULTS
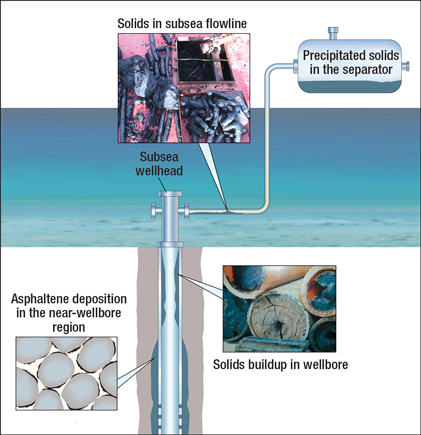
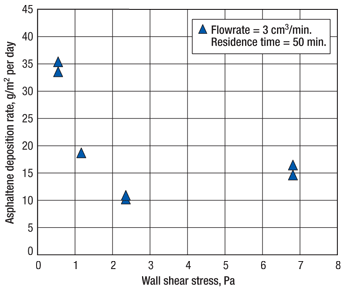
In a four-hour flow-through test, six to seven times more asphaltene was deposited on the flow-through test chamber walls than with the batch test; the batch test had a deposition rate of about 7.5 g/m2 per day. The main learning from the test was that the longer the fluid remains in the cell, the greater the depletion effects will be, Fig. 3. Depletion effects can be quantified through extrapolation by testing deposition at different residence times. Such extrapolation allowed field-scale predictions to be made with accuracy.
Referring to Fig. 3, for both the batch tests and the flow-through tests, more asphaltene was deposited under low-shear-stress conditions (corresponding to a field flowrate of 2,000 bpd) than under high-shear-stress conditions (corresponding to a field flowrate of 8,000 bpd). The simulation of shear stress using the rotating spindle represents the types of changes expected from changes in either flowline diameter or production flowrate. In general, the higher the Reynolds number of the fluid, the higher the shear stress on the deposit and, accordingly, the lower the deposition rate. This relationship has been verified anecdotally from field observations.
 |
|
Fig. 3. The effect of shear stress on the deposition rate of asphaltenes at 69°C, 3,000 psia and a residence time of 50 min. (tests 5–11).
|
|
The relationship between deposition and residence time suggested that deposition may occur mostly at initial contact. One might reason that the longer the asphaltenic oil was in contact with the cell walls, the more asphaltene would be deposited. In fact, deposition amounts do not increase with residence time, giving credence to the hypothesis that long residence times resulted in asphaltene particles that were too large to adhere to the walls and were merely flushed from the cell through the outlet flowline. One can imagine fine droplets of aerosol paint adhering to a slick metal surface, whereas large droplets would tend to run.
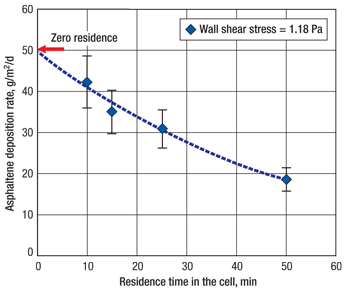
The pressure effect on deposition was also analyzed, Fig. 4. Since it has been well-established that pressure, not temperature, is the parameter most associated with asphaltene deposition in primary recovery, it was deemed appropriate to test to see if other parameter changes interacted with pressure to affect deposition. The quantity of asphaltene deposition was expected to rise as pressure was lowered to the bubble point. No deposition was observed at either shear rate when pressure was held above the asphaltene onset point. A significant increase in deposition mass was observed when the pressure on fluids at high and low shear stress was reduced to the bubble point. The low-shear deposition was more than double that of the high-shear sample. These phenomena will be studied further in the future, but it is safe to conclude that, if higher production rates are maintained, less deposition will occur.
 |
|
Fig. 4. Pressure and shear plots show little to no deposition if pressure is maintained above asphaltene onset pressure; however, high deposition rates are observed when pressure drops to the bubblepoint, and is further accelerated if shear (flowrate) is reduced.
|
|
NO IMPROVEMENT AT HIGHER CONCENTRATION
The tests were concluded by studying the efficacy of an inhibitor used commonly by Shell in a problematic field. While the efficiency of the inhibitor proved to be excellent, it was found that chemical efficiency does not correlate to concentration. The threefold improvement in inhibition was obtained using a 150-ppm solution; yet, when the inhibitor’s concentration was boosted to 500 ppm, no change compared to the 150-ppm test was observed. The observation is expected to have economic benefits because it will discourage the wasting of chemicals, since adding chemicals beyond some threshold has no observable effect on deposition. Further, under high flowrate conditions, it was found that inhibitor efficacy is reduced. Since the tests proved that asphaltene deposition is minimal at high flowrates, perhaps the use of inhibitor chemicals under these conditions could be suppressed altogether.
The above conclusions validated Shell’s asphaltene deposition model and established a clear link between lab testing using the new flow-through cell and actual field applications. 
ACKNOWLEDGMENT
This article was prepared from SPE 124956 presented at the SPE Annual Technical Conference and Exhibition held in New Orleans, Oct. 4–7, 2009.
LITERATURE CITED
1 Zougari, M. et al., “Organic solids deposition and control: Design and application,” Energy & Fuels, 20, 2006, pp. 1656–1663.
|
THE AUTHORS
|
| |
Kamran Akbarzadeh is a Senior Research Scientist and Flow Assurance Research Team Leader at DBR Technology Center, Schlumberger. He has more than 10 years of research experience in flow assurance, with a focus on organic solids precipitation and deposition. Over his five-year career with Schlumberger, Dr. Akbarzadeh has managed many commercial and research projects. He holds a PhD in chemical engineering from Shiraz University, Iran.
|
|
| |
Zhongxin Huo is a Staff Research Engineer with Shell Projects and Technology. He has more than 12 years of industry experience, specifically on asphaltene, wax and hydrate deposition and fluid phase behavior. Over his five years with Shell, Dr. Huo has led the development of an asphaltene deposition model that was validated through field data and successfully applied in various fields. He holds a BS degree in chemical engineering from Tsinghua University in Beijing and a PhD degree in chemical engineering from the Colorado School of Mines.
|
|
| |
George Broze is a Staff Research Engineer with Shell Projects and Technology. He has 25 years of combined research experience in fluid dynamics and flow assurance. He has worked on most of Shell’s deepwater developments in the past 15 years. Currently, Dr. Broze is the Research Team Lead for wax and asphaltenes in Shell’s Deepwater Technology group. He has a BS degree in chemical engineering from Rice University and a PhD in mechanical engineering from the University of Houston.
|
|