Applying biotechnology to increase microbial activity enhances production by 102,900 bbl.
T. N. Nazina, A. A. Grigor’yan, N. M. Shestakova, T. L. Babich, N. K. Pavlova, V. S. Ivoilov, S. S. Belyaev, M. V. Ivanov, Russian Academy of Sciences, Moscow; Q. Feng, F. Ni, J. Wang, Dagang Oilfield Co., China; Y. She, T. Xiang, B. Mei, Yangtze University, China; Z. Luo, Petrochina Co. Ltd., China
Microbiological technology for Microbial Enhanced Oil Recovery (MEOR) based on the activation of the stratal microflora was tested in the high-temperature horizons of the Kongdian reservoir (60˚C) of the Dagang oil field in China. Monitoring the physicochemical, microbiological and production characteristics of the test site revealed changes in the ecosystem as a result of the biotechnology application.
Data on oil stratum microflora are still fragmentary despite the fact that petroleum microbiology was established as a field of science 80 years ago. That fermentative, sulfate-, sulfur- and iron-reducing bacteria, as well as acetogens and methanogens, inhabit oil fields is common knowledge.1 The exploitation of oil fields with waterflooding results in the appearance of oxygen in 1,2 Tests performed in Russian oil fields over the past several years have indicated possible stratal microbial community. This knowledge provides a theoretical basis for the development of a new MEOR biotechnology.3-6
The method consists of the injection of an aerated aqueous solution of nitrogen and phosphorus mineral salts that results in the activation of stratal microflora, primarily oil-oxidizing bacteria responsible for the partial oxidation of residual oil and producing alcohols, fatty acids, surfactants, CO2, etc.2-6 These metabolites and microbial biomass are, in turn, used as substrates by fermentative bacteria and methanogens, which sequentially produce effective oil-releasing agents such as volatile acids, alcohols and gases. In the presence of sulfates, a portion of the oxidized product is used by sulfate-reducing bacteria, which decreases the oil-releasing effect.8
The proposed biotechnology was tested in the oil fields of Bashkortostan, Tatarstan, Western Siberia and Azerbaijan in fields with temperatures ranging from 20-45˚C.5,6 Microbiological methods for MEOR have not been practically applied in high-temperature oil fields.1,7,8 There is also no data in literature on microbiological monitoring of high-temperature oil fields.
The main purpose of this project was to study the possibility of applying biotechnology for MEOR based on stratal microflora activation in high-temperature petroleum reservoirs. This article presents the results of the four year (from 2000-2003) monitoring of the applied biotechnology in the high-temperature Kongdian bed.
MATERIALS AND METHODS
The microbiological method for MEOR was applied in the high-temperature horizons of the North block of the Kongdian bed, Dagang Field, China. Table 1 shows the geological, geochemical, microbiological and production properties of the Kongdian bed, as well as in the previously published works.9-11 The bed has been exploited for about 30 years through flooding with surface fresh water and co-produced water separated from oil to maintain stratal pressure. A total of 22 production and 11 injection wells were located at the test site, which is hydrodynamically isolated from other stratal zones.
TABLE 1. Parameters before the biotechnology application
Click Table to Enlarge. |
 |
|
From 2001-2003, water-air mixture with mineral salts was pumped into the injection wells for two or three days every month between the months of March to September. A total of 15 injection cycles were performed over the three years of the trial, and all of the injection wells at the site were involved in the experiment.
From June 2000 until September 2003, about 40 parameters of injection water and formation water from 22 production wells were monitored. The produced fluids volumes and the proportions of water, oil and gas were measured daily, whereas the physicochemical composition of the formation water and gases from the production wells was analyzed monthly. Microbiological, biogeochemical and rheological parameters of the formation water were determined seven times. The composition of nutrient media, as well as the methods and equipment used, have been previously described.9
MICROBIOLOGICAL PROCESSES
Before the pilot trial of the biotechnology in the Kongdian bed, geological, geochemical, hydrochemical and production characteristics of the bed were analyzed. Results showed that a diverse microbial community with a high metabolic potential inhabits the reservoir, Table 1.9-12 Our comprehensive studies allowed us to test the biotechnology for MEOR based on the activation of the microorganisms inhabiting the oil stratum.
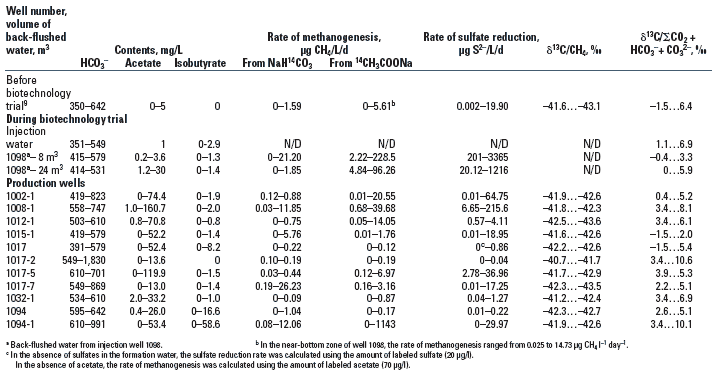
Near-bottom zone of the injection wells. The injected water-air mixture and nitrogen and phosphorus mineral salts stimulated microbial growth in the near-bottom zone of the injection wells. In the near-bottom zone, the matrix contained residual oil and a large amount of microorganisms, and the presence of dissolved oxygen and biogenic elements in the injection water promoted favorable conditions for oil biotransformation. During the trial, the numbers of aerobic organotrophic and hydrocarbon-oxidizing bacteria were highest in the near-bottom zone of the injection wells (e.g., well 1098) and increased from 0-103 to 105 cells/mL and more. The number of fermentative bacteria increased from 102-105 cells/mL to 107 cells/mL, the number of sulfate-reducing bacteria from 0-103 to 105 cells/mL, and the number of methanogens from 0-103 to 106 cells/mL.
Production well zone. The effect of the water-air mixture and mineral salts was well pronounced in 14 production wells, Table 2. In the remaining eight wells, the variation ranges of the numbers of bacteria belonging to all of the studied metabolic groups, of the rates of methanogenesis and of the bicarbonate concentrations in the formation water differed insignificantly from those measured before the experiment. The lack of reaction in the eight production wells can be attributed to their weak hydrodynamic connection with the injection wells.
Aerobic microorganisms. The injected-air oxygen was not detected in the formation water from the production wells; however, the aerobic bacteria in the formation water from the near-bottom zone spread across the stratum. The numbers of aerobic thermophilic organotrophs were as high as 105 cells/mL. Fluctuations in the number of aerobic bacteria and in the amount of their metabolites (volatile acids and carbonates) reached peaks in production wells 1015-1, 1094-1 and 1008-1, where tritium water and 35S were detected earlier and in larger quantities than in other wells in tracer experiments.
All microorganisms that use hydrocarbons as their only source of carbon and energy produce surface-active metabolites that allow insoluble hydrocarbon substrates to penetrate into the bacterial cell.13 It must be emphasized that microorganisms produce surface-active compounds outside of the media with hydrocarbons, since bacterial cultures and biomass always contain biosurfactants as cell-wall components. Hence, even the biomass of the metabolically inactive cells of oil-oxidizing and other bacteria can be used for MEOR.
Anaerobic microorganisms. Thermophilic fermentative bacteria increased from 102-105 cells/mL (before the trial) to 106-109 cells/mL, Table 1. Thermophilic sulfate-reducing bacteria in the water of the majority of the production wells did not change during the experiment, and increased in only four wells.
Before the trial, the sulfate reduction rate in the production wells ranged from 0.002 to 18.9 µg S2-/L/d; in the near-bottom zone of the injection wells it reached 19.90 µg S2-/L/d. During the trial, the most active sulfate reduction process occurred in the near-bottom zone of the injection wells where sulfates were detected. In much of the oil stratum area, the sulfate reduction rate was in the background interval; in ten wells it was 2-15 times higher (up to 147.1-297.1 µg S2-/L/d), Table 2.
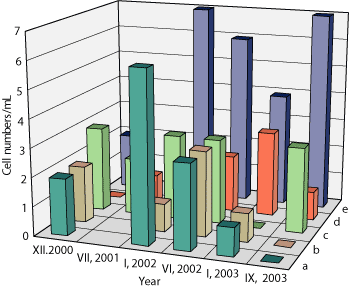
Thermophilic methanogens increased from 102-103 to 104-106 cells/mL in seven wells, Fig. 1. As with sulfate reduction, the methanogenesis rates were high in the near-bottom zone of the injection wells, reaching 229.4 µg CH4 l-1 day-1. Acetate, rather than bicarbonate, was the main methane precursor, which may be attributed to the presence of a large amount of volatile acids during oil biotransformation. In some instances, the rate of methanogenesis estimated with labeled NaH14CO3 was greater than the background values (0-1.59 µg CH4/L/d) and was as high as 3.17-26.23 µg CH4/L/d, Table 2. The maximum rates of methanogenesis estimated with labeled 14CH3COONa in all of the production wells ranged between 6.97 and 1143 µg CH4/L/d, while those measured before the beginning of the experiment were less than 5.61 µg CH4/L/d. An exceedingly high total methanogenesis rate of from acetate and bicarbonate was observed in well 1094-1, where, according to the tracer studies results, water arrived from the injection well with maximum speed.9
 |
|
Fig. 1. Number of microorganisms in the formation water in well 1012-1 during the trial: hydrocarbon-oxidizing bacteria (a), sulfate-reducing bacteria (b), H2-utilizing methanogens (c), acetate-utilizing methanogens (d), fermentative microorganisms (e).
|
|
PHYSICOCHEMICAL PARAMETERS
Pumping the water-air mixture with mineral nitrogen and phosphorus salts stimulated oil biotransformation, which changed the formation water and gas compositions of the Kongdian bed.
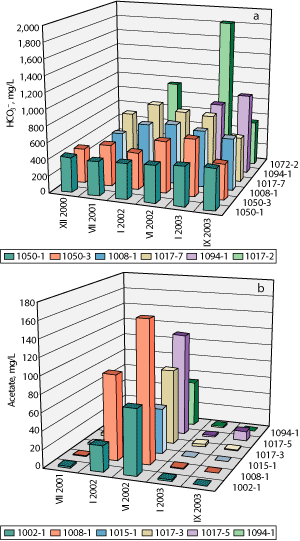
Formation water. The formation water pH increased from 7.2-7.65 to 8.0-9.0 in the majority of the production wells. The concentration of soluble carbonates (HCO3- and CO32- and lower fatty acids, the usual products of microbial oil oxidation, also increased, Table 2, Figs. 2a-b. The biocarbonate content increased by more than 10% of the background values of each well in 10 out of 22 production wells. The concentrations of acetic, formic and isobutyric acids were as high as 160.7, 67.4 and 98.2 mg/L, respectively. At the trial site, the total concentration of acetic, formic and isobutyric acids exceeded 150 mg/L in 15 production wells. In the majority of samples, the background acetate concentration in the formation water was lower than 5 mg/L[9].
 |
|
Fig. 2. Bicarbonate (a) and acetate (b) concentrations in the formation water.
|
|
The concentrations of Ca2+ and Mg2+ did not change during the experiment and were 25-81 and 24-46 mg/L, respectively. As noted above, results showed sulfates in the formation water during the biotechnology test, which were not detected during preliminary investigations. We believe that sulfates were produced as a result of the oxidation of iron sulfides, which were present in the oil stratum, by oxygen from the air entering the stratum with injection water. The sulfate concentration in the formation water increased from 0 to 12-72 mg/L. Ammonium and phosphates introduced into the oil stratum in the form of mineral salts were detected at low concentrations (NH3-N and PO43-; 1-20.8 mg/L and 0-0.86 mg/L, respectively) in almost all of the production wells.
Gas composition. During pilot tests, an increase in the total gas content occurred in the production of the Kongdian bed. The concentrations of carbon dioxide (wells 1015-1, 1017-4 and 1094-1) and methane (wells 1015-1, 1050-3 and 1094-1) increased by 2-4% and 3-5.7%, respectively.
RHEOLOGICAL CHARACTERISTICS
During the biotechnology application, the surface tension of the formation water decreased from 48 to 33.3 mN/m. The average values of interfacial tension against the mixture of
C10-C22 paraffins decreased from 26.6-30.2 to 13.7-25 mN/m, and the average values of dynamic viscosity increased from 0.70 to 0.76 mPa·s. The lowest values of the surface and interfacial tensions of the formation water against paraffins were 29.3-32.4 mN/m (in 11 wells) and 8-10.8 mN/m (wells 1008, 1012 and 1094), respectively. The highest dynamical viscosity of the formation water (0.80-0.83 mPa·s) was detected in wells 1017-2, 1094 and 1017-3.
The values of interfacial tension of the formation water against oil ranged between 2 and 7 mN/m (wells 1002-1, 1012-1, 1017-7 and 1015-1), which were lower than those measured against the C10-C22 paraffin mixture. This may be due to the amphiphilic nature of the biosurfactants that were present at the water-oil interface and in the water-oil emulsion, rather than in the formation water. Oil emulsification by biosurfactants, along with the local rise in the stratal pressure, may be the potential mechanism for MEOR at the test site.
The data correlated with the results of the tracer studies, and confirmed the primary distribution of microorganisms and their metabolites with the formation and injected water in the zones hydrodynamically connected with the injection wells. Moreover, during the biotechnology application, some changes were observed in the water diversion due to penetration of microbial metabolites and biomass. They were confirmed by monitoring production well 1017, which, before the beginning of the experiment, was hydrodynamically isolated from the nearest injection wells 1050 and 1015.9 In the course of the experiment, aerobic bacteria, volatile fatty acids and biosurfactants were observed in this well.
BIOGEOCHEMICAL AND PRODUCTION CHRACTERISTICS
Before the trial, the 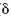 13C values of the carbonates ( 13C values of the carbonates (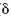 13C/∑CO2 + HCO3- + CO32-) dissolved in the formation water ranged from -1.5 to 6.9‰. During the experiment, the 13C/∑CO2 + HCO3- + CO32-) dissolved in the formation water ranged from -1.5 to 6.9‰. During the experiment, the 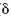 13C values of the carbonates in the formation water of most production wells were the same as before the experiment. In the water from three production wells (1008-1, 1017-2 and 1094-1), isotopically heavy carbonates ( 13C values of the carbonates in the formation water of most production wells were the same as before the experiment. In the water from three production wells (1008-1, 1017-2 and 1094-1), isotopically heavy carbonates (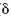 13C 8.1 ... 10.6‰) were detected that were probably produced due to the preferable utilization of isotopically light carbon of carbonates by methanogenic microorganisms observed in these wells, Table 2. The isotopic carbon composition of oil from the Kongdian bed remained unchanged ( 13C 8.1 ... 10.6‰) were detected that were probably produced due to the preferable utilization of isotopically light carbon of carbonates by methanogenic microorganisms observed in these wells, Table 2. The isotopic carbon composition of oil from the Kongdian bed remained unchanged (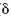 13C -26.5 ... -26.7‰). 13C -26.5 ... -26.7‰).
Before the trial, the 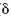 13C value of the methane in the co-produced gas varied within a narrow range, from -41.6 to -43.1‰. During the trial, the total gas concentration increased in a number of wells (1017-2, 1002-1 and 1094-1). Nevertheless, biogenic methane did not cause any considerable changes in the 13C value of the methane in the co-produced gas varied within a narrow range, from -41.6 to -43.1‰. During the trial, the total gas concentration increased in a number of wells (1017-2, 1002-1 and 1094-1). Nevertheless, biogenic methane did not cause any considerable changes in the 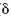 13C value of methane (from -40.5 to -44.4‰), which can be attributed to the fact that the newly generated methane was diluted with the formation gas whose content was high (up to 3.8 m3 of gas/bbl of oil). 13C value of methane (from -40.5 to -44.4‰), which can be attributed to the fact that the newly generated methane was diluted with the formation gas whose content was high (up to 3.8 m3 of gas/bbl of oil).
Earlier, we demonstrated that the biotechnology application at the Kongdian bed resulted in an increase in the number and activity of oil-oxidizing, fermentative and methanogenic microorganisms. In some zones of the stratum, the rate of methanogenesis increased 10-10,000-fold and during oil biodegradation, volatile fatty acids, carbon dioxide, methane, biosurfactants and microbial biomass accumulated. These compounds caused oil emulsification and changes in the rheological characteristics of the formation water, and the local stratal pressure increased due to gas accumulation. The growth of microorganisms in high-permeability zones of the reservoir reduced water mobility in these regions and diverted the water into the regions of the reservoir with higher oil saturation.
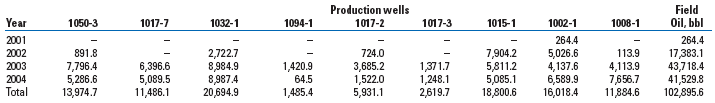
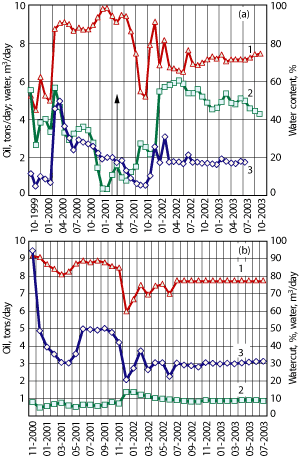
These changes resulted in MEOR in nine wells and in stabilized oil production from the other wells at the test site, Table 3, Fig. 3a-b. For example, oil production from well 1017-2 increased from 25.9-37.1 bpd to 33.6-54.5 bpd. The maximum concentration of bicarbonate (1.8 g/L) and an active population of methanogens (104 cells/mL) that was responsible for a significant increase in gas content were observed in the formation water. Oil production from well 1094-1 increased from 2.1 to 42.3 bpd. In this well, aerobic and anaerobic microorganisms, rates of methanogenesis, concentrations of bicarbonate in formation water and gas in produced oil all increased. An oil production increase accompanied by a water content decrease in the produced liquid, ranging from 13.8 to 42.1% (wells 1017-7, 1017-2, 1008-1 and 1094-1), is proof of the efficiency of the biotechnology. In other production wells, the water content in the produced liquid decreased by no more than 6%. At the same time, the injection wells were operated in a stable regime. As a consequence of the biotechnology application, about 102,900 additional bbl of oil were recovered. After the completion of the biotechnology application, the numbers of microorganisms and the intensity of microbial processes in the stratum decreased.
|
TABLE 3. Additional oil recovery, bbl/yr
Click Table to Enlarge
|
 |
|
 |
|
Fig. 3. Oil and water production and water content in wells 1094-1 (a) and 1008-1 (b) during the trial. The arrow designates the beginning of the injection of the water-air mixture with mineral salts. Lines denote (1) watercut in production, %; (2) oil production, tons/day; and (3) water production, m3/day.
|
|
It was experimentally demonstrated that the high-temperature oil stratum represents an integrated ecosystem in which the microbial community interacts with the abiotic environment in such a manner that the energy flux gives rise to a certain trophic structure. This energy flux is based on the biotransformation of a portion of residual oil in a distinct microbial trophic chain and can be purposefully regulated.
The results demonstrate the high efficacy of the biotechnology for the MEOR based on the activation of the stratal microflora in a high-temperature oil field. 
ACKNOWLEDGMENTS
This work was supported by the Chinese National Petroleum Corporation and the Dagang Oilfield Company (Contract No. DFT04-122-IM-18-20RU), as well as by the Russian Foundation for Basic Research (Projects No. 02-04-39002 and 05-04-39029), Ministry of Education and Science of the Russian Federation (Leading Scientific Schools, grant No. 02.445.11.7409) and the Presidium of the Russian Academy of Sciences (Basic Research Program “Molecular and Cellular Biology”).
LITERATURE CITED
1 Magot, M., Ollivier, B., and B. K. C. Patel, “Microbiology of petroleum reservoirs,” Antonie van Leeuwenhoek, ed., Journal of Microbiology and Serology, 77, 2000, pp. 103-116.
2 Belyaev, S. S., Laurinavichus, K. S., Obraztsova, A. Ya., Gorlatov, S. N., and M. V. Ivanov, “Microbiological processes in the near-bottom zone of injection wells of oil fields,” Mikrobiologiya, 51, 1982, pp. 997-1001.
3 Ivanov, M. V. and S. S. Belyaev, “Microbial activity in waterflooded oil fields and its possible regulation,” International Conference on Microbial Enhancement of Oil Recovery, Shangri, La: Oklahoma, 1983, pp. 48-57.
4 Ivanov, M. V. et al. “Method for development of a flooded oil stratum,” USSR Certificate of Authorship no. 1483944, priority of 11.06.87.
5 Ivanov, M. V. and S. S. Belyaev, “Biotechnology of enhancement of oil recovery based on the geochemical activity of microorganisms (field experiments),” Developments in Petroleum Science, 31, 1991, pp. 421-432.
6 Belyaev, S. S., Borzenkov, I. A., Nazina, T. N., Rozanova, E. P., Glumov, I. F., Ibatullin, R. R., and M. V. Ivanov, “Use of microorganisms in the biotechnology for the enhancement of oil recovery,” Mikrobiologiya, Vol. 73, No. 5, 2004, pp. 590-598.
7 Lazar, I., “MEOR field trials carried out over the world during the last 35 years,” Developments in Petroleum Science, 31, 1991, pp. 485-530.
8 McInerney, M. J, Nagle, D. P, and R. M. Knapp, “Microbially enhanced oil recovery: Past, present, and future,” in Ollivier, B. and M. Magot, eds., Petroleum Microbiology, ASM Press, Washington, DC, 2005, pp. 215-237.
9 Nazina, T. N. et al, “Microbiological investigations of high-temperature oil strata of the Kongdian bed in connection with field tests of a biotechnology for enhancement of oil recovery,” Mikrobiologiya, Vol. 76, No. 3, 2007, pp. 287-296.
10 Nazina, T. N. et al, “The phylogenetic diversity of aerobic organotrophic bacteria from the Dagang high-temperature oil field,” Mikrobiologiya, Vol. 74, No. 3, 2005, pp. 336-342.
11 Nazina, T. N. et al, “Phylogenetic diversity and activity of anaerobic microorganisms of high-temperature horizons of the Dagang oilfield (P. R. China),” Mikrobiologiya, Vol.75, No. 1, 2006, pp. 55-65.
12 Nazina, T. N. et al, “Geobacillus jurassicus sp. nov., a new thermophilic bacterium isolated from a high-temperature petroleum reservoir, and the validation of the Geobacillus species,” Systematic and Applied Microbiology, 28, 2005, pp. 43-53.
13 Ron, E. Z. and E. Rozenberg, “Natural role of biosurfactants,” Environmental Microbiology, 3, 2001, pp. 229-236.
|
THE AUTHORS
|
| |
From the Winogradsky Institute of Microbiology at the Russian Academy of Sciences in Moscow, Tamara N. Nazina is a lead scientist with a PhD in microbiology from Moscow State University, is a Doctor of Biology and is a Laureate of the Russia Government. Dr. Nazina is the author of over 100 papers, and her interests include petroleum microbiology and MEOR studies. Alexander A. Grigor’yan is a junior research scientist, with a PhD in microbiology from the Russian Academy of Sciences. Natalya M. Shestakova is a junior research scientist, and has an MS from Perm State University and a PhD in microbiology from the Russian Academy of Sciences. Tamara L. Babich is a junior research scientist, and has a PhD in microbiology from Moscow State University. Nadezda K. Pavlova is a junior research scientist with an MS in zoology from Moscow State University. Valeriy S. Ivoilov is a research scientist at with an MS in microbiology from Moscow State University. Sergey S. Belyaev is the head of the petroleum microbiology lab ratory and a professor, and has a PhD in microbiology and a Doctor of Biology from Moscow State University. Mikhail V. Ivanov is an Academician and is the head of the microbial biogeochemistry and biogeotechnology department, with a PhD and Doctor of Biology from Moscow State University.
|
|
| |
From the Dagang Oilfield Co., Qingxian Feng is a senior engineer in the EOR department, and has a BS from the Petroleum University of China. Fangtian Ni is a chief engineer and a professor, and has a BS from Nankai University. Jianqiang Wang is an engineer and vice director of the petroleum geology department of the No. 6 oil production plant, and has a BS from Tianjin University.
|
|
| |
From Yangtze University in China, Yuehui She is an associate professor of chemical and environmental engineering, and graduated from the Petroleum University of China, and Hunan Normal University. Tingsheng Xiang is an associate professor, and has a PhD in bioscience from HuaZhong Agriculture University. Bowen Mei is a professor and has BS and MS degrees in geochemistry.
|
|
| |
Zhibin Luo is the head of Chinese work with PetroChina Co. Ltd. in Beijing, and has a BS from the Petroleum University of Beijing.
|
|
|