Oil Country Tubular Goods
Factors identified in environmentally assisted cracking of tubulars
Extensive testing has found that incompatibility of some tubulars and clear brine fluids can lead to substantial cracking failures in high-pressure, high-temperature completions.
Dr. Jeffrey McKennis and Nam-Sook Bae, Tetra Technologies, Inc., The Woodlands, Texas
General corrosion has been substantially eliminated by using corrosion-resistant alloy (CRA) tubulars, but the problem of failures caused by environmentally assisted cracking (EAC) grows with the increase in high-pressure, high-temperature (HP/HT) completions.1 – 3 What started as a research effort to identify the compatibility of JFE Steel Corp. (JFE) and Tetra Technology, Inc. (Tetra) products has provided greater understanding of factors involved in stress corrosion cracking (EAC) .
Examination of field failures has revealed interesting conclusions:
- External stress has not been found to be a significant factor.
- In nearly all cases, failures resulted from EAC. Cracking began from the tubing’s outer surface, rather than from the inside.
These revelations have focused attention on the interaction between the CRA metallurgy and clear brine fluid (CBF) in the annulus.
More than 3,000 tests clearly show that small differences, including contaminants in metal composition and fluid chemistry, substantially impact corrosion and cracking susceptibility. Tubulars and fluids used in today’s HP/HT wells are not generic. Universally held beliefs about fluids, e.g., the safety of bromides and the dangers of chlorides, are not substantiated by the alliance’s extensive testing.
INFORMATION VACUUM
Despite numerous papers about field failures, laboratory testing and other technical studies, a review of the issues of CRA metallurgy and packer fluid chemistries used in HP/HT applications finds a serious lack of empirical data. This deficiency is to be expected, given the variables involved, and the industry’s limited experience with these exotic fluid/ alloy combinations.
Many elements have been seen as contributing to the problems:
- Characteristics of alloys used
- Makeup of fluids, including salt concentrations
- Presence of contaminants, either from fluid and tubing manufacture or from the annulus
- Additives
- Stresses
- Temperature.
This reality exists because broad-scope, multi-condition testing of CRA/CBF combinations had not been done previously. Producers simply lacked specific scientific information for minimizing EAC. Indeed, anecdotal observations, extrapolation of limited data and standard corrosion tests have not provided the technical data necessary to predict how CRA tubing and CBFs will interact in the well. Historically, indicated sources of cracking events have often been more associative than causative.
Thiocyanate, for example, has been implicated in many recorded failures. However, in the Erskine failure, thiocyanate’s presence probably did not cause the failure. Most likely, oxygen entry into a highly concentrated calcium chloride solution was the culprit. Indeed, several field failures have occurred with thiocyanate-free packer fluids.3 With such widespread uncertainty, a dedicated research effort has clearly been needed.
JOINT RESEARCH EFFORT
This issue centers on interaction of packer fluids and CRA tubing. Until now, minimal empirical data have been available to help producers design completions. This inadequacy is noteworthy, given the large downside of a catastrophic failure.
To remedy this lack of information, JFE and Tetra, respective manufacturers of martensitic (martensite is the hard constituent of quenched steel) stainless tubulars and clear brine fluids, joined in a research project called the ChemiMetallurgy™ alliance. This alliance has undertaken a massive analysis and testing program to scientifically quantify factors contributing to EAC in HP/HT wells. With the help of Intercorr International, Inc., a recognized, independent corrosion laboratory, the alliance has developed a testing regimen to replicate mechanisms observed in many recorded failures. This replication duplicates real world conditions to exhaustively test typical metallurgies and fluids in HP/HT wells.
TESTING TO REAL WORLD CONDITIONS
The test protocol uses C-rings cut from stock tubing that are machined only on the ID to obtain uniform wall thickness. The C-rings are stressed well beyond normal NACE or API levels and immersed in various fluids, along with typical contaminants and additives. Autoclave/ oven test systems enable extensive testing that includes CBFs of various chemistries and densities, appropriate additives, numerous CRA metallurgies and typical contaminants.
Tests are conducted over a 100°-to-400°F range to simulate harsh, variable conditions encountered in HP/HT wells. By the end of first-quarter 2005, more than 3,000 tests had been conducted, involving more than 20 fluid combinations, six metallurgies, and an array of additive and contaminant packages.
Upon completion of each test, C-rings are visually and microscopically examined to determine their condition, and to categorize them as either passing or failing, Fig.1. C-rings that exhibit cracking, pitting or other severe localized corrosion are analyzed to determine the mechanisms involved. Many are sent to JFE’s research facility in Japan for analysis by metallurgists to identify elements in the failure.
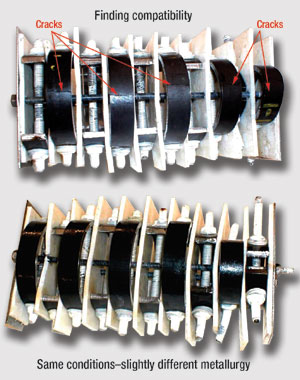 |
Fig. 1. Two slightly different Cr steels stress-tested under the same real world conditions in CaCl2 at 300°F with typical contaminants and additives, yielding substantially different results.
|
|
Such analysis has proven vital in understanding what causes cracking. These in-depth studies have shown surprising results. Rather than stemming from one mechanism, failures are often driven by two or more factors. For example, sulfide stress cracking (SSC) of martensitic CRAs, which normally occurs at low temperatures, may coincide with halide stress cracking at temperatures much higher than normally associated with SSC. Such observations give important guidance in predicting tubing/ fluid compatibility, substantially mitigating the failure potential.
In fact, EAC, which involves sulfide and halide stress cracking, requires interaction of three domains: 1) Sensitive metallurgy; 2) A corrosive environment; and 3) Stress, Fig. 2.4 Many CRA metals used in HP/HT applications have sensitive metallurgy, while CBFs are corrosive. Stress, can be either internal – from manufacturing, e.g., annealing, cold working, etc., – or external, due to operations or improper handling. If any of these domains is absent, cracking will not occur.
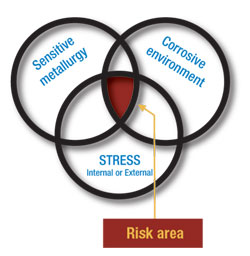 |
Fig. 2. Three domains are required for environmentally assisted cracking.
|
|
SIGNIFICANT REVELATIONS
Although the study is ongoing, testing to date invalidates some widely held beliefs.
Are these fluids and metallurgies generic? Conventional wisdom alleges that CBFs and CRAs are generic, that all manufacturers’ brines or tubulars are chemically or metallurgically equivalent. After more than 3,000 tests, a very different reality has emerged. There are significant differences between products because of variations in raw materials, manufacturing processes, quality control, product formulation and composition. Even small differences can play a major role in the potential for cracking. The chemistry of the total fluid in the well determines how tubing and fluid will react.
Are bromides best? Although many field failures studied involved fluids containing chloride, studies show that bromides are not always better than fluids containing chloride. Failures have been observed with both sodium and calcium bromide fluids at elevated temperatures. In many uses, fluids containing chloride with appropriate additive packages can be as reliable as fluids containing bromides.
Is oxygen always a problem? Studies show that eliminating oxygen is necessary to avoid EAC.2 Yet, tests show that oxygen in several different, inhibited fluid combinations was less detrimental than expected, even at 300° to 400°F. In these tests, 13Cr95 suffered significant pitting but no cracking. HP2 13Cr110 had no significant pitting or cracking.
Are concentrated zinc fluids corrosive? Because concentrated zinc bromide fluids are acidic, they are thought to be causative to EAC, particularly where high stresses can induce hydrogen embrittlement or cracking. Tests show this to be false. A number of blended zinc fluids at various densities show marked resistance to cracking, even at 300°F to 400°F.
COMPLETION DESIGN/ COST IMPLICATIONS
Test results put new tools in the producers’ hands. Most of the typical combinations of chrome tubulars and CBFs have been tested for compatibility, so producers can access the alliance database to determine combinations best able to handle anticipated well conditions. With this predictability, producers can select the most economical tubing/ fluid combinations at minimum risk. Moreover, the database helps to enhance existing products and generate new HP/HT products. 
LITERATURE CITED
1 Mowat, D.E., M. C. Edgeton and E. H. R. Wade, “Erskine field HPHT workover and tubing corrosion failure investigation,” SPE paper 67779, 2001 SPE/IADC Drilling Conference, Amsterdam, The Netherlands, Feb. 27 – Mar. 1, 2001.
2 Mack, R., C. Williams, S. Lester and J. Casassa, “Stress corrosion cracking of a cold worked 22Cr duplex stainless steel production tubing in a high-density, clear brine CaCl2 packer fluid – Results of the failure analysis at Deep Alex and associated laboratory experiments,” Corrosion 2002, paper 02067, NACE International, Houston, Texas, 2002.
3 Ibrahim, M.Z., N. Hudson, K. Selamat, P. S. Chen, K. Nakamura and M. Ueda, “Corrosion behavior of Super 13 martensitic stainless steels in completion fluids,” Corrosion 2003, paper 03097, NACE International, Houston, Texas, 2003.
4 A preliminary summary of some of these findings has been presented at the AADE 2005 National Technical Conference and Exhibition, April 5 – 7, 2005, Houston, Texas, AADE paper 05-NTCE-16.
THE AUTHORS
|
 |
Dr. Jeffrey McKennis manages technology services and product development at Tetra Technologies, Inc., supervising the development and application of completion fluids and additives. He has conducted research activities for more than 25 years, in the oil and gas industry and in academia. Dr. McKennis holds a BS degree in chemistry/ mathematics from Yale University and a PhD in chemistry from the University of Michigan.
|
|
 |
Nam-Sook Bae is a senior chemist working in product development at Tetra Technologies, Inc. She has over 15 years of experience in analytical methodology, and in the formulation of new products and technologies. She holds a BS degree in biology/ chemistry from Sogang University, South Korea.
|
| |
|
|